Biomarkers capable of providing rapid diagnosis of severe bacterial infections (SBIs), establishing their prognosis, monitoring their course and helping to decide the earliest moment to withdraw antibiotics without the risk of a relapse would be a great help in clinical practice. Nearly 200 molecules have been proposed as potential markers, though only 20% have reached the point of being evaluated in appropriate studies. The latest research focuses on the fields of genomics and metabolomics, and the application of solutions based on nanotechnology to prevent, detect and treat SBIs has begun to be considered.1 While we await the results of these studies, we need to optimise the use of the markers most commonly employed in clinical practice: C-reactive protein (CRP), procalcitonin (PCT) and interleukin 6 (IL-6).
Studies such as the one published in this issue of Anales de Pediatría by Parada et al.2 evaluating the impact of PCT on the management of hospitalised febrile infants, are greatly needed. As the authors point out, the retrospective nature of the study, the small number of patients with SBIs, 75% of them with urinary infections, and the administration of antibiotics to patients without SBIs weaken the conclusions. Nevertheless, their findings alert us to the importance of analysing the value (or lack of value) that markers add to clinical practice. In contrast, in a recent multicentre study on 2047 febrile infants Milcent et al.3 found that PCT (cut-off value: 0.3ng/mL; area under curve: 0.91; 95% CI: 0.83–0.99) was superior to CRP (cut-off value: 2mg/dL; area under curve: 0.75; 95% CI: 0.65–0.89) for detecting invasive bacterial infection and that the two markers were similar for detecting SBI. Their results suggest that these markers could enhance clinical practice in febrile infants. We will try to analyse the limitations and strengths of markers so as to understand the contradictory results of many published studies and attempt to get the most out of them.4
Early diagnosis and treatment of SBIs influence their prognosis. The first limitation of CRP, PCT and IL-6 is that their levels rise in the presence of any inflammatory response, both when the cause is infection and when it is trauma, severe shock or a situation involving tissue necrosis (postoperative complications, major burns, etc.). Therefore when we have an elevated result, we have to decide whether there is some non-infectious cause to explain that rise or whether, on the contrary, we should begin antibiotic treatment immediately. If the result is low, it is unlikely that there is an SBI, although it is vital to take the kinetics of these markers into account to avoid asking for them at times when a low level is of no value, either because it is too soon for them to have started rising or because it is too late for them to have remained elevated. IL-6 rises in the first 2–3h after the insult, PCT between 6 and 12h and CRP between 24 and 48h. Therefore, to perform an early diagnosis and decide on an immediate antibiotic treatment the ideal marker would be IL-6, followed by PCT. IL-6 sometimes falls very rapidly and could therefore give us a false negative if the request is late, and CRP is more useful if at least 24h have elapsed since the onset of symptoms.
The cut-off values are set depending on how sensitive and specific we wish the test to be. We must treat our patients individually and use the marker as one more aid in the decision, establishing whether we want to prioritise sensitivity, as in the case of immunodepressed patients, in whom we prefer to start antibiotics rather than running the risk of delayed treatment. The normal value for PCT in the healthy population, discounting the presence of inflammation, is less than 0.1ng/mL. However, in the published studies cut-off values of between 0.2 and 0.5ng/mL are established for SBI in previously healthy patients, 1–2ng/mL for SBI in intensive care patients and 2–5ng/mL for postoperative patients. The same is true for the other inflammatory markers. The cut-off values also vary depending on the information we want to obtain from the marker, with different values for trying to predict the risk of mortality or for indicating withdrawal of antibiotics.
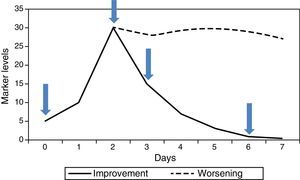
In hospitalised patients it is the kinetics of successive values, rather than one isolated value, that have most to contribute. Generally there are 4 useful values (Fig. 1): (1) initial value of the marker (this will be the one that helps to confirm or rule out the diagnostic suspicion), (2) maximum value (marks the worst point in the course of the disease), (3) falling value (confirms good response to treatment), (4) value below which we consider withdrawing the antibiotics (end of the process). PCT has been the most studied for monitoring this course, since its kinetic is suitable. It begins to rise quite rapidly (6–12h), reaches its maximum value in 24–48h, and if progress is favourable it begins to fall from that point, halving its previous value every 24h. CRP is also useful, although its slower kinetics cause it to rise and fall about 24h later. IL-6 tends to fall very rapidly and is therefore not useful for monitoring the course of the infection, nor as a criterion for withdrawal of antibiotic treatment. If the disease progresses unfavourably, the marker levels do not fall but remain raised for a period of time (Fig. 1), indicating a possible need for a change of therapy, specifically in antibiotic treatment, while awaiting blood cultures, in the case of infection.
In the last few years a number of studies have been published in which markers are used as a guide for withdrawal of antibiotic treatment. PCT has been the object of several trials and meta-analyses. The levels proposed for suspending antibiotics are below 0.25ng/mL, or between 0.25 and 0.50ng/mL provided they are below 80% of the maximum value reached.5 CRP may also be suitable for this purpose.
Other markers that may provide information for decisions on the prognosis and admission of patients are being investigated. The use of panels of biomarkers has been described as a solution that benefits from the advantages and reduces the limitations of an isolated marker. However, greater methodological rigour is needed in clinical studies with markers to demonstrate the cost-effectiveness of such combinations.
To sum up, Parada et al.3 have highlighted the need to assess the impact of introducing biomarkers into clinical practice. Issues such as proper training of medical staff before instituting a diagnostic test, appropriate selection of the patients for whom the request is to be made, interpretation of the results in the clinical context of each patient and making people aware that all tests have a cost must be kept firmly in mind. We should not routinely request a marker without having considered beforehand, after an exhaustive clinical assessment, what approach we will adopt according to the result we receive. Requesting a large number of laboratory tests is not synonymous with better clinical practice, but rather the opposite.
Conflicts of interestCorsino Rey has received funding from the Brahms and Thermofisher companies to give lectures at conferences on subjects related to biomarkers and sepsis.
Please cite this article as: Rey Galán C. Biomarcadores de infección bacteriana grave: ¿ayudan en la práctica clínica?. An Pediatr (Barc). 2016;84:247–248.