Acute otitis media (AOM) is common in children aged <3 years. A pneumococcal conjugate vaccine (PCV) (PCV7; Prevenar, Pfizer/Wyeth, USA) has been available in Spain since 2001, which has a coverage rate of 50–60% in children aged <5 years.
Materials and methodsChildren aged ≥3 to 36 months with AOM confirmed by an ear-nose-throat specialist were enrolled at seven centers in Spain (February 2009–May 2012) (GSK study identifier: 111425). Middle-ear-fluid samples were collected by tympanocentesis or spontaneous otorrhea and cultured for bacterial identification. Culture-negative samples were further analyzed using polymerase chain reaction (PCR).
ResultsOf 125 confirmed AOM episodes in 124 children, 117 were analyzed (median age: 17 months (range: 3–35); eight AOM episodes were excluded from analyses. Overall, 69% (81/117) episodes were combined culture- and PCR-positive for ≥1 bacterial pathogen; 44% (52/117) and 39% (46/117) were positive for Haemophilus influenzae (Hi) and Streptococcus pneumoniae (Spn), respectively. 77 of 117 episodes were cultured for ≥1 bacteria, of which 63 were culture-positive; most commonly Spn (24/77; 31%) and Hi (32/77; 42%). PCR on culture-negative episodes identified 48% Hi- and 55% Spn-positive episodes. The most common Spn serotype was 19F (4/24; 17%) followed by 19A (3/24; 13%); all Hi-positive episodes were non-typeable (NTHi). 81/117 AOM episodes (69%) occurred in children who had received ≥1 pneumococcal vaccine dose.
ConclusionsNTHi and Spn were the main etiological agents for AOM in Spain. Impact of pneumococcal vaccination on AOM requires further evaluation in Spain, after higher vaccination coverage rate is reached.
La otitis media aguda (OMA) es común en niños menores de 3 años. En España hay disponible una vacuna neumocócica conjugada (VNC) (VNC7; Prevenar, Pfizer/Wyeth, EE. UU.) desde 2001, habiéndose alcanzado una cobertura vacunal del 50-60% en niños menores de 5 años.
Materiales y métodosSe reclutó a niños de 3 a 36 meses con OMA confirmada por especialista en otorrinolaringología en 7 centros españoles (febrero 2009-mayo 2012) (Proyectoe GSK: 111425). Se obtuvieron muestras de exudado del oído medio mediante timpanocentesis o de otorrea espontánea, y se hizo cultivo para identificación bacteriana. En muestras con cultivos negativos se realizó análisis adicional mediante reacción en cadena de la polimerasa (PCR).
ResultadosDe 125 episodios de OMA confirmados en 124 niños, se analizaron 117 (edad mediana: 17 meses [rango: 3–35]); 8 episodios de OMA fueron excluidos del análisis. En total, combinando resultados de cultivo y PCR, se identificaron uno o más patógenos bacterianos en el 69% (81/117) de los episodios; identificándose Haemophilus influenzae (Hi) en el 44% (52/117) y Streptococcus pneumoniae (Spn) en el 39% (46/117). En 77 de los 117 episodios se hizo cultivo para uno o más patógenos, resultando positivo en 63, con mayor frecuencia para Spn (24/77; 31%) e Hi (32/77; 42%). La PCR en episodios con cultivos negativos detectó Hi en el 48% y Spn en el 55% de las muestras. El serotipo de Spn más común fue el 19F (4/24; 17%) seguido del 19A (3/24; 13%); todos los episodios en los que se identificó Hi correspondieron a Hi no tipificable (HiNT). Un total de 81/117 episodios de OMA (69%) se presentaron en niños que habían recibido una o más dosis de vacuna antineumocócica.
ConclusionesHiNT y Spn resultaron ser los principales agentes etiológicos de la OMA en España. Para conocer el impacto de la vacunación antineumocócica en la OMA en España harán falta estudios adicionales cuando se haya alcanzado un nivel de cobertura mayor.
Globally, acute otitis media (AOM) is one of the most common childhood infections affecting over 75% of children younger than 3 years of age1 and representing the most common condition for antibiotic prescription in young children.2 Approximately 709 million cases of AOM occur throughout the world each year, of which 51% occur in children younger than 5 years of age.3
In Spain, the annual incidence of AOM in children below 2 years of age is estimated at 392 per 1000 person-years, as compared with 263 per 1000 person-years in children aged between 3 and 5 years.4 Antibiotics are prescribed as first-line treatment in more than 90% of Spanish children presenting with symptoms of AOM (e.g. ear discharge), although an initial observation is recommended beforehand.5
Following the introduction of the 7-valent pneumococcal conjugate vaccine (PCV) (PCV7; Prevenar 7, Pfizer/Wyeth, USA) onto the Spanish private market in 2001,6 the vaccination coverage rate in children younger than 5 years had reached 50–60% by 2007.7 Other licensed PCV formulations have since been introduced in Spain, including the 10-valent PCV (PCV10; Synflorix™, GSK Vaccines, Belgium) in 2009 and 13-valent PCV (PCV13; Prevenar 13, Pfizer/Wyeth, USA) in 2010.7
Before the introduction of PCV, Streptococcus pneumoniae (Spn) and Haemophilus influenzae (Hi) were the most important etiological bacterial agents for AOM in Spanish children.8 However, the underlying microbiology of infectious diseases changes over time as a consequence of vaccination and antibiotic consumption9 and several global studies have documented a decrease in the circulation of pneumococcal vaccine serotypes and an increase in non-vaccine serotypes and Hi serotypes since PCV7 implementation.10 This study was designed to assess the bacterial etiology of AOM in Spanish children younger than 3 years of age using both culture and polymerase chain reaction (PCR); it complements a previous study describing the bacterial etiology of recurrent AOM and AOM treatment failures in Spanish children during the post-PCV era.7
Material and methodsStudy design and participantsThis prospective, epidemiological study was conducted at seven centres across Spain between February 2009 and May 2012 (GSK study identifier: 111425). Children aged ≥3 to 36 months with ear–nose–throat specialist-confirmed AOM, and who could provide a middle ear fluid sample (MEF), were enrolled in the study. MEF samples were collected by tympanocentesis; in case of otorrhea, the samples were collected via deep aspiration of MEF through needle insertion after ear canal cleaning.
AOM was confirmed based on the acute and sudden onset of the disease within the previous 3 days: fever, irritability, intense erythema of the tympanum or earache; and presence of effusion in the middle ear. The presence of middle ear effusion was characterized by tympanum bulging, limited or no mobility in the tympanum (evaluated using pneumatic otoscopy), fluid behind the tympanum and/or otorrhea (defined as perforation occurring within 48h before enrollment).
Children were excluded from participating in the study if they were hospitalized during the AOM episode (to exclude possible confusion with intrahospital infections) or in the event of otitis externa/otitis media with effusion: AOM for >72h or otorrhea for >48h before enrollment; or in situ transtympanic aerators. Children who received antibiotics for illnesses other than AOM during the 72-hour period before enrollment and were prescribed antibiotics before performing tympanocentesis were also excluded. Finally, patients with protocol-forbidden medical conditions were excluded.
The study adhered to the principles of Good Clinical Practice, including the 1964 Declaration of Helsinki and local Spanish rules and regulations. The study was approved by the Institutional Review Board of each participating center and parents/guardians provided written informed consent before enrollment.
Bacterial identification and antibacterial susceptibility testingMEF samples were collected by tympanocentesis performed under otoscopy through a reusable CDT Aspirator attached to a CDT speculum, or by sampling of spontaneous otorrhea. In cases of bilateral AOM, MEF samples were collected from both ears. Tympanocentesis was performed without anesthesia. Aspirated MEF samples were inoculated onto Amies transport medium11 and kept at room temperature for 16h (and up to a maximum of 48h) before subsequent analysis at a GSK-designated laboratory. MEF samples were then inoculated onto chocolate (with bacitracin for otorrhea samples) and blood agar (with nalidixic acid). Bacterial identification was undertaken using standard bacteriological procedures.12 Serotyping for Spn was performed by Quellung's reaction and for Hi using monovalent anti-sera.
Culture-negative samples were further analyzed using real-time PCR assay for the presence of the pneumococcal pneumolysin (ply) gene,13 pneumococcal wzg gene,14 and the omp2 gene for non-capsulated and capsulated Hi.15 Inhibited PCR reactions were all checked using a commercial exogenous internal positive control (Applied Biosystems, Foster City, CA, USA). A subsequent extraction for eliminating inhibitors was performed on inhibited samples using 20% (w/v) Chelex-100 resin (BioRad Laboratories, Hercules, CA, USA). The samples were further tested for antibacterial susceptibility. Minimal inhibitory concentrations were derived using Etest (bioMérieux, France) and interpreted with the criteria published by the Clinical and Laboratory Standards Institute in 2009.16
Statistical analysesAnalyses were performed on children meeting all the selection criteria, complying with protocol-defined procedures, and from whom laboratory results of the MEF sample were available. The proportion of AOM episodes caused by Spn, Hi and other bacterial pathogens and the percentages of Spn and Hi serotypes were calculated. Only the first AOM episode was included and any recurrent AOM cases were excluded from analyses. The antibacterial susceptibility of Spn and Hi was determined. History of previous PCV, Hi-type B and influenza vaccinations was recorded. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
ResultsStudy participants and demographicsOf 125 AOM episodes in 124 subjects, recorded during the study period, 117 episodes (tympanocentesis=91; otorrhea=26) (from 117 subjects) were analyzed. Only one subject had two AOM episodes, of which one episode was eliminated from the analyses. Therefore, a total of eight AOM episodes were excluded from the analyses: protocol violation (n=1), having a protocol-forbidden medical condition (n=4) and missing MEF/otorrhea sample (n=3). The median age of children was 17 months (range: 3–35) and 60.7% were male. AOM episodes were most frequently observed in children aged 11–22 months (46.2%; 54/117) followed by 23–35 months (32.5%; 38/117) and 3–10 months (21.4%; 25/117). At least one dose of a pneumococcal vaccine and Hi-type B vaccine was received by 69.2% and 95.7% subjects, respectively. None of the subjects received influenza vaccine. Table 1 presents information on age groups in the study, summary of vaccination history and antibiotic treatment.
Characteristics of enrolled children.
| Characteristics | Categories | n (%) |
|---|---|---|
| Age groups (N=117) | ||
| Age (months) | 03–10 | 25 (21.4) |
| 11–22 | 54 (46.2) | |
| 23–35 | 38 (32.5) | |
| Summary of vaccination history (N=117) | ||
| Child received at least one dose of a | Yes | 81 (69.2) |
| Pneumococcal vaccine | No | 36 (30.8) |
| Child received at least one dose of a Haemophilus influenzae-type B vaccine | Yes | 112 (95.7) |
| No | 3 (2.6) | |
| Unknown | 2 (1.7) | |
| Child received at least one dose of a Influenza vaccine | Yes | – |
| No | 116 (99.1) | |
| Unknown | 1 (0.9) | |
| Antibiotic treatment (N=117) | ||
| Antibiotics taken before the sample was obtained | Yes | – |
| No | 117 (100) | |
| Antibiotics taken within the past one month | Yes | 7 (6.0) |
| No | 110 (94.0) | |
N=total number of subjects.
n (%)=number (percentage) of subjects in each group.
The microbiological results are reported in Table 2. Bacterial culture tests were performed for 117 episodes. Growth for any bacterium was cultured from 66% (77/117) of episodes: 82% of which (63/77) were culture-positive for at least one bacterium under study (Spn, Hi, Streptococcus pyogenes [Spy] or Moraxella catarrhalis [Mcat]). The most frequent bacteria were Hi (42%; 32/77) and Spn (31%; 24/77). Spy was isolated in 10 samples and Mcat in 1 sample; co-infection was detected in 10 episodes, of which 4 co-infections involved bacterium under study: Spn and Hi (n=3); Spy and Hi (n=1). The remaining 40 episodes were culture-negative. However, PCR was performed for 42 episodes (40 culture-negative episodes+2 culture-positive episodes), of which 48% (n=20) were found to be Hi-positive and 55% (n=23) were Spn-positive.
Bacterial identification of AOM episodes by culture and PCR.
| Culture (n=77) | PCR (n=42) | Combined culture+PCR (n=117) | |
|---|---|---|---|
| n′ (%) | n′ (%) | n′ (%) | |
| Positive for at least 1 pathogenic bacteria | 63 (82) | 29 (69) | 81 (69) |
| Streptococcus pneumoniae | 24 (31) | 23 (55) | 46 (39) |
| Haemophilus influenzae | 32 (42) | 20 (48) | 52 (44) |
| Streptococcus pyogenes | 10 (13) | – | – |
| Moraxella catarrhalis | 1 (1) | – | – |
| Co-infection with Streptococcus pneumoniae and Haemophilus influenzae | 3 (4) | 14 (33) | 17 (15) |
n=number of episodes analyzed according to culture, PCR or both.
n′ (%)=numbers (percentage) of episodes positive for each bacterium.
There were two AOM episodes that were tested by both culture and PCR.
Note: Culture-negative episodes were not tested for Streptococcus pyogenes and Moraxella catarrhalis by PCR.
AOM, acute otitis media; PCR, polymerase chain reaction.
Combined culture and PCR results indicated that 52 AOM episodes (44%) were Hi-positive and 46 (39%) were Spn-positive; 17 episodes (15%) were positive for both Hi and Spn.
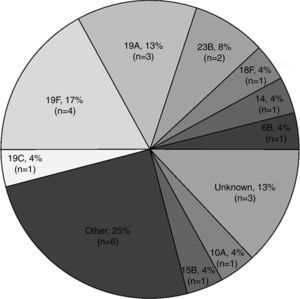
The most common Spn serotypes amongst culture-positive episodes were 19F (17%; 4/24) and 19A (13%; 3/24) (Fig. 1). In episodes from children who had been previously vaccinated, PCV7 vaccine serotypes (14 and 19F) were present in 19% (3/16) of episodes and PCV7/PCV13 vaccine serotype (6B) was present in 6% (1/16) of episodes (Table 3). PCV7 vaccine serotype (19F) was present in 25% (2/8) episodes from unvaccinated children.
Serogroups and serotypes of Streptococcus pneumoniae-positive episodes (by culture) by name of pneumococcal conjugate vaccination status (N=16).
| PCV name | Serotypes | Vaccinated total (N=16) |
|---|---|---|
| n (%) | ||
| PCV7 | 14a | 1 (6) |
| 19A | 3 (19) | |
| 19C | 1 (6) | |
| 19Fa | 2 (13) | |
| Other (8, 15, 21, 35) | 4 (25) | |
| Unknown | 2 (13) | |
| PCV13 | Other (21) | 1 (6) |
| PCV7 and PCV13 | 6Ba | 1 (6) |
| PCV10 | Other A (10A) | 1 (6) |
| Serotypes | Unvaccinated total (N=8) |
|---|---|
| n (%) | |
| 18F | 1 (13) |
| 19F | 2 (25) |
| 23B | 2 (25) |
| Other (22) | 1 (13) |
| Other B (15B) | 1 (13) |
| Unknown | 1 (13) |
N=number of Streptococcus pneumoniae-positive episodes vaccinated and unvaccinated with pneumococcal conjugate vaccine.
n (%)=number (percentage) of Streptococcus pneumoniae-positive episodes in each category.
All 32 Hi-positive episodes were non-typeable (NTHi): 69% (n=22) in vaccinated (PCV vaccine) and 31% (n=10) in unvaccinated (PCV vaccine) children.
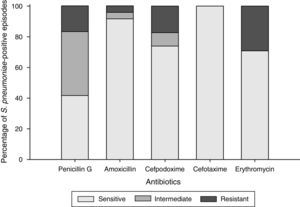
Antibacterial susceptibilityOf the 24 Spn-positive episodes, all (100%) were susceptible to cefotaxime, 22 (92%) to amoxicillin, 17 (71%) to erythromycin and 17 (74%) to cefpodoxime (since there was one missing Spn-positive episode susceptible to only cefpodoxime, 23 of 24 Spn-positive episodes were considered for the analyses). Ten episodes were susceptible and intermediately susceptible to penicillin G and four (17%) were resistant to penicillin (Fig. 2). Serotype 19A was most commonly resistant to penicillin G, amoxicillin, cefpodoxime, and erythromycin.
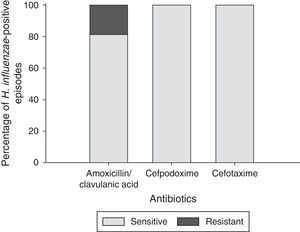
Of the 32 Hi-positive episodes, all (100%) were susceptible to both cefpodoxime and cefotaxime and 26 (81%) to amoxicillin/clavulanic acid. Six episodes (19%) were resistant to amoxicillin/clavulanic acid (Fig. 3).
Multi-drug resistance was observed in 17% (4/24) Spn-positive episodes: one episode was resistant to penicillin G, cefpodoxime and erythromycin; and three episodes were resistant to penicillin G, amoxicillin, cefpodoxime and erythromycin. Multi-drug resistance was observed in six NTHi-positive episodes (19%).
Microbiological results in relation to vaccination statusOf the 81 children positive for at least one pathogenic bacteria by combined culture+PCR, 55 (68%) children had received the appropriate PCV doses according to their age: 61% (11/18) aged 3–10 months, 64% (21/33) aged 11–22 months and 77% (23/30) aged 23–35 months. The vaccines administered corresponded to PCV7 in 62 children (77%, where 44 children [71%] received ≥3 doses), PCV10 in nine (11%, where six children [67%] received ≥3 doses) and PCV13 in 17 children (21%, where two children [12%] received ≥3 doses). There was no statistically significant difference in terms of vaccination between Hi-positive (P=1.000) and Spn-positive children (P=0.9497).
DiscussionThis study identified and characterized the bacterial pathogens associated with AOM in Spanish children less than 3 years of age during the post-PCV era. We found a relatively high percentage (69%) of episodes culture-positive for at least one pathogenic bacterium by combined culture and PCR. Hi and Spn were the most common pathogens isolated from AOM episodes. This finding is in agreement with previous studies conducted in Mexico, Columbia, Venezuela, Germany and Spain that have implicated these pathogens as the main causative agents of AOM.17–21 Interestingly, similar proportions of Spn and Hi detected by culture and PCR highlight the relevance of PCR as an effective detection method. This is corroborated by a former study that identified Spn and Hi in culture-negative samples using PCR-based diagnosis.22
Spn and NTHi are highly responsible for the vast number of AOM cases throughout the world, which has not changed significantly in the past fifty years before or after PCV introduction.17,23 Widespread vaccination with PCV could result in a lower role in AOM for Spn. This could in turn result in NTHi becoming a more important pathogen in the years to come.23
At least one dose of a Hi-type B vaccine was received by 112 children (95.7%). In our study, NTHi was responsible for all Hi-positive episodes, as has previously been observed in etiology studies where the majority or all Hi-positive episodes were non-typeable.17,18,20,24 Vaccination with Hi-type B could result in a lower incidence of Hi-type B as pathogen causing AOM, but has not been able to reduce the incidence of NTHi. The most common pneumococcal serotype was 19F followed by 19A in this study. An earlier study from Spain also found 19F as the predominant serotype followed by 23F which was not found in this study.21 The persistence of 19F is noteworthy and underscores the lack of vaccine efficacy against this serotype in Spain. Although serotype distribution varies geographically, studies in Spain and several other countries have emphasized the predominance of serotype 19A after PCV introduction.21,25–27 The presence of 19A in this study possibly indicates insufficient cross-protection against this serotype by PCVs. Although Spn and NTHi are the main causative pathogens of AOM, and with Spn being the less predominant pathogen, some papers have recently highlighted a shifting of pathogens, with Spn being less frequent than NTHi in young children, in concordance with our data.23,28
As more than 80% of physicians prescribed an antibiotic as initial treatment for patients with AOM in Spain,5 high antibiotic resistance is expected.29 However, in this study we observed that the majority of Spn and Hi-positive episodes were susceptible to amoxicillin and amoxicillin/clavulanic acid, respectively. This finding supports the continued use of amoxicillin or amoxicillin/clavulanic as the first line of AOM treatment in Spain.7 Also, all Spn- and Hi-positive episodes were susceptible to cefotaxime. Our antibacterial susceptibility patterns are similar to previous findings in Spain21 and may be helpful when considering antibiotic management in Spain.
The AOM cases in this study may not be representative of all AOM cases in the Spanish population and the results should be considered as merely descriptive. Although tympanocentesis is an effective method for identifying bacterial pathogens from the middle ear, it is not routinely performed in Spain and contributed to our relatively small enrollment numbers and inability to draw strong conclusions. Furthermore, our study excluded recurrent AOM cases and included only the first AOM episode, which could be another limiting factor given that in Spain, it is difficult to perform tympanocentesis on the first AOM episode.
Our study shows that in this post-PCV era, NTHi and Spn were the leading bacterial etiological agents of AOM in young Spanish children, and shifting pathogens has been observed where NTHi has emerged as an important pathogen of AOM. Further studies are needed to evaluate the impact of pneumococcal vaccination on AOM disease burden, after higher vaccination coverage rate is reached.
TrademarksPrevenar 7 and Prevenar 13 are registered trademarks of Pfizer/Wyeth.
Synflorix™ is a registered trademark of the GSK group of companies.
FundingThis study (GSK study identifier: 111425) was sponsored by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and publishing of the manuscript.
AuthorshipAll authors had full access to the data and were involved in revising the manuscript critically for important intellectual content, and gave final approval. The corresponding author had final responsibility to submit for publication. A confidentiality agreement was set between the authors and the sponsor.
Conflict of interestSistiaga-Hernando A, García-Corbeira P, McCoig C and Devadiga R are employees of the GSK group of companies. Sistiaga-Hernando A, García-Corbeira P and McCoig C report ownership of stock options/restricted shares from the GSK group of companies. Pumarola F reports payments received from the GSK group of companies for congress registration fees. Gómez Martínez JR and Iniesta Turpin J report investigator fees received by their institution from the GSK group of companies during the conduct of the study. Salamanca de la Cueva I, Moraga-Llop FA, Cardelús S and Rosell Ferrer R report no conflict of interest.
The authors would like to thank Ashmita Ravishankar and Mark Franco for medical writing (Publications Writer, GSK), Julia Donnelly (freelance Publications Manager on behalf of GSK Vaccines) for editorial support and Jérémie Dedessus Le Moutier and Grégory Leroux (Publications Manager, Business and Decision Life Sciences on behalf of GSK Vaccines) for coordination in the development of the manuscript.
Please cite this article as: Pumarola F, Salamanca de la Cueva I, Sistiaga-Hernando A, García-Corbeira P, Moraga-Llop FA, Cardelús S, et al. Etiología bacteriana de la otitis media aguda en españa en la era de la vacuna neumocócica conjugada. An Pediatr (Barc). 2016;85:224–231.
Upon presentation orally at the National Congress of the Spanish Society of Otorhinolaryngology and Cervical-Facial Pathology (SEORL-PCF) held in Madrid (Spain) from 25 to 28 October 2013.