The use of procalcitonin (PCT) in the evaluation of the febrile infant in the emergency care unit has been widespread. The aim of this study is to assess whether the introduction of PCT has changed the management of hospitalised febrile infants and the cost/effectiveness of this marker.
Materials and methodsA retrospective study was performed comparing 2 periods: January–December 2009 (without PCT) and January–December 2011 (routine use of PCT). Infants aged 7–90 days with fever who were admitted to a university hospital and had a blood test performed were included in the study. Bacterial infection rate, antibiotic use, hospitalisation days, and analytical costs were compared. Evaluations were made using PCT, C-reactive protein (CRP), white cell count, Rochester score, and the lab-score proposed by Galetto-Lacour for the diagnosis of bacterial infection.
ResultsA total of 109 patients were included in period 1, and 111 in period 2 (87 of which had a PCT value). The prevalence of bacterial infection, use of antibiotics, number of blood tests, and days of hospital admission was similar in both periods. The blood test cost was significantly higher in the second period. Sensitivity, specificity, positive predictive value and negative predictive value were 70.6, 58.1, 52.6 and 75%, respectively for the CRP (cut-off 1mg/dL) and 41.7; 78.4; 57.7, and 65.6% for the PCT (cut-off value 0.5ng/mL).
ConclusionsThe use of PCT does not seem to have a significant impact on the management of the hospitalised febrile infant.
El uso de la procalcitonina (PCT) se ha generalizado en la evaluación del lactante febril en urgencias. Este trabajo pretende evaluar si la introducción de la PCT ha cambiado el manejo del lactante febril hospitalizado y el coste/efectividad de dicho marcador.
Pacientes y métodosEstudio retrospectivo comparando 2 periodos: enero-diciembre de 2009 (sin PCT) y enero-diciembre de 2011 (uso rutinario de PCT). Se incluyó a los pacientes de 7 a 90 días de vida con fiebre ingresados en un hospital universitario y con analítica realizada. Se compararon porcentajes de infección bacteriana, uso de antibióticos, estancia hospitalaria y coste analítico. Se evaluó la PCT, la proteína-C reactiva (PCR), recuento leucocitario, score de Rochester y el lab-score propuesto por Galetto-Lacour para el diagnóstico de infección bacteriana.
ResultadosSe incluyó a 109 pacientes en el periodo 1 y 111 en el periodo 2 (de los cuales en 87 se dispuso de valour de PCT). La prevalencia de infección bacteriana, uso de antibióticos, repetición de analíticas y estancia hospitalaria fue similar en los 2 periodos. El coste analítico fue significativamente superior en el segundo periodo. La sensibilidad, especificidad, valour predictivo positivo y valour predictivo negativo de la PCR (punto de corte 1mg/dl) fue 70,6; 58,1; 52,6 y 75% y de la PCT (punto de corte 0,5ng/ml) 41,7; 78,4; 57,7 y 65,6%.
ConclusionesEl uso de la PCT no parece haber tenido un impacto significativo en el manejo del paciente hospitalizado.
Fever is one of the most common reasons for paediatric medical visits. Most episodes of fever are due to benign and self-limiting viral infections that do not require treatment. However, in infants, and especially in those aged less than 3 months, fever can be a sign of severe bacterial infection (SBI) and thus results in a significant number of admissions. The assessment of the febrile infant is a diagnostic challenge that has led to the development of different tools that attempt to identify the patients most at risk of SBI: clinical scores such as the Yale scale,1 blood markers assessed alone (C-reactive protein [CRP], procalcitonin [PCT], interleukin-6,…) or in combination (lab-score2) as well as combinations of clinical and laboratory criteria (Rochester criteria3).
The determination of PCT may offer some advantages in the early diagnosis of bacterial infection due to its shorter half-life and more rapid elevation, and it seems that it is a more specific marker than CRP in children with SBI.4 Most studies agree that low levels of PCT have a high negative predictive value (NPV) for ruling out bacteraemia, and in the past decade PCT has been included in many protocols for the management of children with fever without source (FWS) in emergency settings. Nevertheless, testing of PCT levels may also lead to false positive and false negative results (sensitivity [Sn], 70–91%; specificity [Sp], 59–85%),5–7 which means that clinical decisions cannot be based on this marker alone.
The usefulness of PCT as a predictive marker has been validated in several studies in adults, and its use to guide initiation or discontinuation of treatment in adult patients seems safe and efficacious, and contributes to a significant reduction in antibiotic use.8,9 However, few studies have evaluated the results of the routine use of PCT in clinical practice (indication of admission, repetition of laboratory tests, antibiotic use, length of stay) and its cost in paediatrics.
The aim of this study was to assess whether determination of PCT has changed the assessment and management of hospitalised febrile infants in our hospital, and to compare the Sn, Sp, positive predictive value (PPV) and NPV of different markers and scores in this population. We also assessed the added cost of determining CRP and PCT levels compared to traditional combined clinical and laboratory scores.
Materials and methodsWe conducted a retrospective descriptive study over two time periods: January to December 2009 (period 1: no PCT) and January to December 2011 (period 2: routine use of PCT). The protocol for the management of febrile infants aged less than 3 months was the same during the two periods except for the routine use of PCT in period 2: in our hospital, the protocol for infants aged 1–3 months with fever without source is based on the Rochester criteria; for newborns, the protocol includes blood, urine and cerebrospinal fluid (CSF) testing, chest X-ray in case of respiratory manifestations, hospital admission, and decision to use or not use antibiotics based on clinical manifestations and test results; and in patients with specific diagnoses (bronchiolitis, urinary tract infection [UTI], pneumonia, etc.) the corresponding protocol was applied. The protocol did not include any recommendations regarding determination of PCT.
We included patients aged 7–90 days admitted to our university hospital with fever (defined as axillary temperature≥37.5°C). We excluded patients that had not undergone blood testing. We collected the following data: demographic data, clinical manifestations, complete blood count, baseline and peak CRP, baseline and peak PCT, urine sediment test (US) or urine test strip (UTS), Rochester score, lab-score (CRP+PCT+UTS) calculated with the baseline test results, blood culture (BC), urine culture (UC), length of stay, antibiotic use and number of tests ordered. Other tests performed in select cases were CSF culture, detection of respiratory viruses by polymerase chain reaction or of respiratory syncytial virus (RSV) antigen in nasopharyngeal aspirate samples, and chest radiograph.
We defined SBI according to the criteria established by Maniaci et al.10 which we can summarise as:
- •
Definite SBI: focal bacterial infection found during physical examination (other than otitis media), or bacteraemia, UTI, meningitis, bacterial pneumonia or bacterial gastroenteritis defined respectively as: positive BC to a pathogen; UC of catheter specimen with more than 50,000CFU of a single microorganism or between 10,000 and 49,000 CFU if accompanied by abnormal US (>5 white blood cells [WBC] per high-power field [hpf]) or abnormal UTS (positive for nitrites or WBCs); positive CSF culture or bacteraemia with pleocytosis in CSF; positive pleural fluid culture or radiographic findings compatible with bacterial pneumonia accompanied by sputum or blood culture positive to a respiratory pathogen; and last of all, positive stool culture.
- •
Possible SBI: UC of catheter specimen with 10,000–49,000CFU/mL of a single pathogen with normal US and UTS; or radiography compatible with pneumonia but without bacteriological confirmation by blood, sputum, or pleural fluid culture.
- •
No SBI: all other cases.
When multiple organisms were found in the UC, the case was considered a definite UTI if there were more than 50,000CFU of a single dominant pathogen, and a possible ITU if there were at least 50,000CFU of multiple pathogens with no particular dominance, regardless of the US results.
We obtained the data on testing costs from the laboratory of our hospital as of December 2013: complete blood count, 4.29euro/panel; CRP, 3.68euro/determination; PCT, 20.15euro/determination.
We conducted the analysis for the overall sample and for patient subsets: patients with good general health, patients with FWS, patients with normal US/UTS results, and patients aged less than 1 month. We calculated the Sn, Sp PPV and NPV of PCT and CRP levels for different cut-off values (PCT≥0.26, ≥0.5, ≥1, ≥2ng/mL; CRP≥1, ≥2.75, ≥4, ≥10mg/dL), Rochester criteria,3 US (cut-off values: ≥5 and ≥10WBC/hpf) and lab-score (cut-off value, ≥3)2,11 for the diagnosis of SBI. In cases in which UTS results were not available, we used the US results to calculate the lab-score.
We assessed whether the systematic determination of PCT was associated with increased or decreased antibiotic use, changes in length of stay, and repetition of laboratory tests.
We considered that antibiotic treatment was appropriate when antibiotics were prescribed to patients diagnosed with a SBI (possible or definite) and when they were not prescribed to patients without a SBI.
We collected data from patient charts and laboratory records in a spreadsheet (Microsoft Office Excel 2007) and analysed them using the SPSS software version 15.0 for Windows. We used the chi square test to compare dichotomous variables and the Student t test or the Mann–Whitney U test to compare quantitative variables (α=0.05). We computed ROC curves to assess the diagnostic yield of CRP, PCT and leukocytosis in cases of possible or definite SBI using the data for period 2 of patients for whom PCT results were available.
ResultsThe study included 220 patients: 109 in period 1 and 111 in period 2; PCT results were only available in 87 patients in period 2. Of the 220 patients, 78 (35.4%) were less than 30 days’ chronological age, and 80 (36.4%) had FWS. Table 1 summarises the baseline demographic, clinical and laboratory characteristics, which were not significantly different between the two periods.
Clinical and laboratory characteristics in the two periods.
| Period 1 n=109 | Period 2 with PCT n=87 | P | |
|---|---|---|---|
| Male: n (%) | 64 (58.7) | 55 (63.2) | .521 |
| Age (days)a | 9–90; 44.0 (23.9) | 10–90; 45 (23.9) | .765 |
| Length of stay (days)a | 1–13; 5.0 (2.6) | 2–11; 4.80 (2.19) | .594 |
| Fever<24h, n (%) | 97 (89.0) | 74 (85.1) | .534 |
| Clinical manifestationsb, n (%) | .507 | ||
| Respiratoryc | 53 (48.6) | 36 (41.4) | |
| Cutaneous | 2 (1.8) | 5 (5.7) | |
| Gastrointestinald | 26 (23.8) | 16 (18.4) | |
| Other, n | – | 1 (1.1) | |
| Without a source | 38 (34.9) | 35 (40.2) | |
| Baseline WBC/mm3(e) | 11,260 (6553) | 12,034 (5696) | .392 |
| Baseline bands/mm3(e) | 144 (524) | 73 (173) | .195 |
| Baseline CRP (mg/dL)e | 2.3 (4.0) | 2.73 (3.42) | .426 |
| Baseline PCT (ng/dL)e | – | 1.0 (2.8) | – |
| Abnormal sediment n (%) | 18 (16.5) | 16 (18.4) | |
| Low-risk Rochester n (%) | 45 (41.3) | 39 (44.8) | .618 |
| Number of test per patient, n (%) | .893 | ||
| 1 | 63 (57.8) | 53 (60.1) | |
| 2 | 33 (30.3) | 25 (28.7) | |
| >2 | 13 (11.9) | 9 (10.3) | |
| Antibiotic therapy | 69 (63.3) | 57 (65.5) | .748 |
| Possible or definite UTI | 33 (30.3) | 28 (32.2) | .774 |
| Possible or definite SBI | 41 (37.6) | 35 (40.2) | .592 |
| Definite SBI | 26 (23.9) | 27 (31.0) | .185 |
A SBI was diagnosed in 85 patients (38.6% of the total sample). Although the percentage of patients admitted with a SBI (possible or definite) was slightly higher in the period when PCT was routinely used (37.6% in period 1 compared to 40.2% in period 2 in patients with PCT results), the difference was not statistically significant (P=.59).
The prevalence of SBI (possible or definite) was similar in patients aged less than 1 month (39.4%) and more than 1 month (37.5%) (P=.77), and most cases corresponded to UTIs.
A total of 42 patients in period 1 and 28 patients with available PCT results in period 2 were aged less than 1 month. The prevalence of possible or definite SBI in this subset of patients in the two periods was 35.5% and 39.2%, respectively (P=.76). We also found no statistically significant differences in antibiotic use between the two periods (66.6% and 78.6%; P=.28).
The number of laboratory tests performed and the percentage of patients treated with antibiotics were similar in both periods (Table 1). When we compared the length of stay of patients in period 1 with that of patients with PCT results in period 2 (mean stay, 6 and 2.2 days, respectively) the difference was not statistically significant, either (P=.71).
Table 2 lists the diagnoses of patients with possible or definite SBI in both periods.
Diagnosis of patients with possible or definite SBI in both periods.
| Period 1 n=41 | Period 2 with PCT n=36 | |
|---|---|---|
| Bacteraemia | 1 | 3 |
| Enterococcus | 1 | |
| N. meninigitidis | 1 | |
| S. agalactiae | 1 | |
| S. aureus | 1 | |
| Meningitis E. coli+UTI | 1 | |
| GEA Campylobacter | 1 | |
| ITU | 33 | 27 |
| Pneumonia | 5 | 2 |
| Mastitis | 1 | |
| Omphalitis | 1 | 1 |
| Suppurative otitis media | 1 |
Of the four patients with bacteraemia (one in the pre-PCT period and three with PCT results in period 2) all had presented with fever of less than 12h duration. Two had leukopaenia, all had CRP levels above 1mg/dL (6.9mg/dL, 1.4mg/dL, 1.5mg/dL and 8.9mg/dL) and their PCT levels were 0.24ng/mL, 0.38ng/mL and 1.07ng/mL.
Table 3 shows the Sn, Sp, PPV and PPV of the different clinical and laboratory parameters (for different cut-off values) in the diagnosis of possible or definite SBI. The most sensitive criterion was the Rochester score; the most specific was a CRP level equal or greater than 10mg/dL (although >1500bands/mm3, PCT≥2ng/mL and abnormal US had very similar specificities). The parameter with the best PPV was a CRP level equal or greater than 10mg/dL (100%), while the Rochester criteria had the highest NPV (76.8%). The highest Youden indices were 0.424 for a CRP value of 2.35mg/dL, and 0.345 for a PCT value of 0.351ng/mL.
Sensibility, specificity, predictive values, likelihood ratios (LRs) and Youden indices for different parameters for SBI (possible or definite) for the entire sample of patients (n=220).
| Sn as % | Sp as % | PPV as % | NPV as % | LR+ | LR− | Youden | |
|---|---|---|---|---|---|---|---|
| WBC (<5000 or >15,000) | 37.6 | 69.7 | 44.4 | 63.7 | 1.19 | 0.91 | 0.07 |
| Bands (>1500/mm3) | 0 | 98.5 | 0 | 60.5 | 0.00 | 1.02 | −0.015 |
| Rochester | 74.4 | 54.5 | 51.2 | 76.8 | 1.64 | 0.47 | 0.289 |
| Abnormal urine sedimenta | 39.0 | 97.4 | 91.4 | 69.9 | 15.00 | 0.63 | 0.364 |
| Abnormal urine sedimentb | 43.9 | 92.4 | 80.0 | 70.5 | 5.78 | 0.61 | 0.363 |
| Baseline CRP (≥1mg/dL) | 70.6 | 58.1 | 52.6 | 75.0 | 1.68 | 0.51 | 0.287 |
| Baseline CRP (≥2.75mg/dL) | 49.4 | 86.0 | 70.0 | 72.1 | 3.53 | 0.59 | 0.354 |
| Baseline CRP (≥4mg/dL) | 34.1 | 93.0 | 76.3 | 68.2 | 4.87 | 0.71 | 0.271 |
| Baseline CRP (≥10mg/dL) | 9.4 | 100 | 100 | 62.6 | - | 0.91 | 0.094 |
| Baseline PCT (≥0.26ng/mL)c | 66.7 | 51.0 | 49.0 | 68.4 | 1.36 | 0.65 | 0.177 |
| Baseline PCT (≥0.5ng/mL)c | 41.7 | 78.4 | 57.7 | 65.6 | 1.93 | 0.74 | 0.201 |
| Baseline PCT (≥1ng/mL)c | 30.6 | 90.2 | 68.8 | 64.8 | 3.12 | 0.77 | 0.208 |
| Baseline (≥2ng/mL)c | 19.4 | 98.0 | 87.5 | 63.3 | 9.70 | 0.82 | 0.174 |
| Lab-score (≥3)c | 45.7 | 93.9 | 84.2 | 70.7 | 7.49 | 0.58 | 0.396 |
The analysis of the subset of patients whose management is the most challenging (FWS and normal US) yielded similar results (Table 4). Of the 24 patients with FWS and a normal US for which CRP and PCT results were available, and applying the cut-off values proposed for the lab-score11 (CRP≥4mg/dL, PCT≥0.5ng/mL), two of the five patients with possible or definite SBI had negative results for both markers.
Sensibility, specificity, predictive values and likelihood ratios (LRs) in patients with fever without source and normal urine sediment results (<10 leukocytes/high-power field) for possible or definite SBI (n=61).
| Sn as % | Sp as % | PPV as % | NPV as % | LR+ | LR− | |
|---|---|---|---|---|---|---|
| Baseline WBC (<5000 or >15,000) | 44.4 | 73.8 | 42.1 | 75.6 | 1.69 | 0.75 |
| Baseline bands (>1500/mm3) | 0 | 100 | 0 | 70.0 | 1.00 | |
| Rochester | 66.7 | 58.1 | 40.0 | 80.6 | 1.59 | 0.57 |
| Baseline CRP (>1mg/dL) | 52.9 | 64.3 | 37.5 | 77.1 | 1.48 | 0.73 |
| Baseline CRP (>4mg/dL) | 29.4 | 97.6 | 83.3 | 77.4 | 12.25 | 0.72 |
| Baseline CRP (>10mg/dL) | 0 | 100 | 0 | 71.2 | 1.00 | |
| Baseline PCT (>0.26ng/mL)a | 50.0 | 57.9 | 27.3 | 78.6 | 1.19 | 0.86 |
| Baseline PCT (>0.5ng/mL)a | 33.3 | 84.2 | 40.0 | 80.0 | 2.11 | 0.79 |
| Baseline PCT (>1ng/mL)a | 33.3 | 89.5 | 50.0 | 81.0 | 3.17 | 0.75 |
In the subset of patients aged less than 1 month (n=28), the NPV of PCT (cut-off value, 0.5ng/mL) was 75%: a SBI was diagnosed in five out of the twenty patients with PCT values of less than 0.5ng/mL.
The analysis of the subset of patients with FWS (n=80) showed that 35 patients (43.8%) had a possible or definite SBI (94% of which were UTIs). Table 5 presents the clinical and laboratory characteristics of the patients with SBI compared to those of patients with no SBI. The baseline CRP, PCT and WBC levels and the peak CRP level were significantly different in patients with SBI compared to patients without SBI, but this was not the case of the peak PCT level, which was similar in both groups.
Characteristics of patients with fever without source classified by SBI diagnosis (n=80).
| No SBI | Possible or definite SBI | P | |
|---|---|---|---|
| N | 46 | 34 | |
| Male: n (%) | 24 (52.2) | 24 (70.6) | .097 |
| Age (days) | 37.8 (23.6) | 46.8 (25.9) | .061 |
| Length of stay (days) | 4.76 (2.39) | 6.12 (1.95) | .022 |
| Fever <24h: n (%) | 45 (97.8) | 31 (91.2) | .177 |
| WBC/mm3 | 8877 (4979) | 12,496 (7328) | .047 |
| Bands/mm3 | 28 (95) | 87 (168) | .031 |
| Baseline CRP (mg/dL) | 1.02 (1.45) | 3.69 (4.25) | <.005 |
| Peak CRP (mg/dL) | 1.24 (1.63) | 3.97 (4.18) | <.005 |
| Baseline PCT (ng/mL)a | 0.39 (0.46) | 2.54 (5.66) | .031 |
| Peak PCT (ng/mL)b | 1.09 (2.69) | 4.74 (13.52) | .094 |
| Abnormal sediment, n (%) | 2 (4.4) | 16 (47.1) | <.005 |
| Low-risk Rochester, n (%) | 26 (56.5) | 6 (17.6) | <.005 |
| N tests, n (%) | |||
| 1 | 23 (50.0) | 22 (58.8) | |
| 2 | 17 (37.0) | 8 (23.5) | |
| >2 | 6 (13.0) | 6 (17.6) | |
| Antibiotic treatment, n (%) | 27 (60.0) | 34 (97.1) | <.005 |
| Appropriate treatment, n (%) | 19 (41.3) | 34 (100) | <.005 |
| Possible or definite UTI, n (%) | 0 | 32 (94.1) | <.005 |
| Definite SBI, n (%) | 0 | 27 (79.4) | <.005 |
Values for age, length of stay, leukocytes, bands, CRP and PCT expressed as mean (SD).
Antibiotic treatment was appropriate in 72.5% of patients in period 1 and 73.6% of patients with PCT results in period 2, and this difference was not statistically significant.
The laboratory costs for patients in period 2 were triple the costs for patients in period 1 (period 1 mean, 11.1euro/patient [SD, 4.95]; period 2 mean, 38.7euro/patient with PCT [SD, 16]; P=.000).
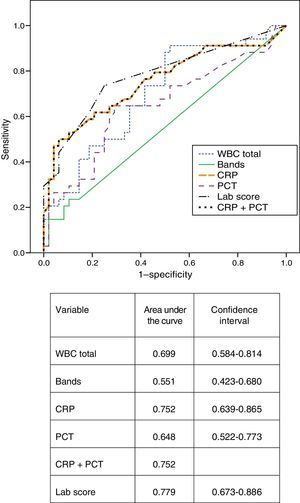
Fig. 1 presents the ROC curves obtained for CRP, PCT, leukocytosis, bands, CRP and PCT combined, and the lab-score. The area under the curve for CRP was greater than the area under the curve for PCT (0.752 and 0.648, respectively) although the difference was not statistically significant (P=.196). The area under the curve for the lab-score was slightly greater than the areas under the curve for CRP alone or CRP combined with PCT, but these differences were not statistically significant, either (P=.399).
DiscussionThe PCT test was introduced in our hospital in 2010. At first it was only used in select cases, but starting in 2011 it came to be used routinely in the assessment of febrile infants. The results of this study show that the routine use of PCT has not changed the management of hospitalised patients aged less than 3 months.
Most of the published studies on the use of PCT in the paediatric population have been conducted in patients with FWS in emergency settings.5,6,12,13 These studies support the usefulness of PCT as a predictive marker of potentially severe bacterial infection (bacteraemia, sepsis, sepsis of urinary tract origin and severe pneumonia) under these circumstances.
The predictive values of PCT obtained in our study differed significantly from those obtained in series that included patients aged less than 36 months treated in emergency settings7: in our study, the NPV ranged between 63% and 81% for the different PCT cut-off values (0.26ng/mL; 0.5ng/mL; 1ng/mL), far from the NPVs nearing 100% obtained in other studies. This could be due to the fact that our study only included hospitalised patients: the selected sample had a higher prevalence of SBI than samples in studies carried out in emergency departments (higher percentage of patients aged <1month, patients not meeting the low-risk Rochester criteria, etc.). The prevalence of definite SBI in our study was 31.3%, similar to the 26.4% prevalence in the study by Bressan et al.11 but considerably higher than the prevalence found in studies like the one conducted by Maniaci et al.10 (9.7%).
The multicentric study by Fernández-López et al.14 compared different markers in 352 children aged 1 to 36 months with FWS in an emergency setting, and concluded that the best marker is PCT with a cut-off value of 0.53ng/mL (Sn, 65.5%; Sp, 94.3%). In our study, a similar cut-off value (PCT=0.5ng/mL) corresponded to a Sn of only 41.7%, and a Sp of 78.4%.
As for CRP, we observed that when we applied cut-off value of 1mg/dL, the Sn reached 70% and the NPV was 75% (higher than those obtained with PCT), although the PPV and the Sp were low (75% and 58.1% respectively). In fact, of the 12 patients admitted with FWS, a normal US and a CRP of less than 1mg/dL, only one had a SBI (UTI) with a baseline PCT of 0.18ng/mL. Of the 24 patients with FWS and a normal US, none received a diagnosis of definite or possible SBI with a CRP value below 1mg/dL and a PCT value above 0.5ng/mL.
When we analysed the lab-score proposed by Galetto-Lacour et al. in 2008, we obtained results similar to those published by Bressan et al.11 with a Sn of 45.7% and a Sp of 93.9% using a cut-off value of 3, so this score also does not seem to offer clear advantages over the CRP alone.
A meta-analysis published in 20105 concluded that the main advantage of PCT is ruling out the presence of SBI. Consequently, we would expect a lower number of admissions or a higher percentage of patients with a final diagnosis of SBI in the period in which PCT was available. The fact that this was not observed in our study may be explained by the sample not being large enough to obtain statistically significant results, or the lack of confidence in this marker of the paediatricians responsible for admitting the patients: 19 of the 87 patients admitted during period 2 had FWS, normal US results and PCT levels below 0.5ng/mL.
In recent years, multiple studies have been published that determined optimal PCT cut-off values for diagnosing SBI, differentiating between viral and bacterial disease, and differentiating between invasive and noninvasive bacterial infection (pyelonephritis vs. cystitis).15,16 The established cut-off values range between 0.12ng/mL and 1ng/mL. This variability may be one of the factors contributing to the general lack of confidence in this marker.
Buendia and Colantonio17 applied decision analysis and developed a structured cost-effectiveness economic model to compare the use of PCT, CRP and the Rochester criteria for the diagnosis of SBI in infants aged less than 3 months, and demonstrated the higher cost associated with the use of PCT. In our study, we found that laboratory costs tripled in the period when PCT was ordered compared to the first period. Since there were no differences between both periods in the percentage of patients admitted with a final diagnosis of SBI and the percentage of patients treated with antibiotics was also similar, it did not seem, in our case, that the use of PCT led to a reduction in health care costs.
Our findings were also consistent with the results of other studies that have assessed the impact of the use of PCT: a prospective study by Lacroix et al.18 that compared the use of the lab-score with that of traditional markers (CRP, WBC and bands) did not find differences in the associated antibiotic use and hospital admissions.
The fear that continuing to add markers (without removing any of those already in use) could lead to an increase in the number of admissions and courses of antibiotic treatment that result from a single elevated marker (that is, admitting patients based only on an elevated PCT level) was not supported by our results: in period 1, 13 patients were admitted with FWS and a low risk of SBI based on the Rochester criteria, while 18 patients were admitted in period 2 (11.9% vs. 16.2%; P=.36). The number of patients with FWS, low-risk based on the Rochester criteria and a CRP of less than 1mg/dL was similar in both periods (9 and 10 patients, respectively).
The weaknesses of our study are its retrospective design, the small number of patients with FWS, and the inability to confirm the absence of SBI in patients categorised as no SBI that had received antibiotic treatment.
Despite the lesser scientific value of retrospective studies, we believe that assessing and sharing the outcomes of introducing new diagnostic tools in everyday practice is essential to the development of good clinical practice.
In short, in our hospital, the use of PCT in the management of fever in hospitalised infants aged less than 3 months did not lead to a reduction in antibiotic use, number of tests performed, or length of stay. We also found no evidence of improvement in the selection of patients for admission (similar SBI percentages in the period when PCT was routinely used and in the period without PCT), although this finding should be confirmed by performing a prospective study with random allocation of PCT testing versus no PCT testing and assessment of the difference in the percentage of patients admitted to hospital.
Biomarkers provide information that supplements the clinical assessment, but they may also carry significant added costs, not only due to the cost of the tests themselves, but also because they may lead to additional treatments or hospital admissions. When it comes to infants aged less than 3 months admitted with fever, in whom UTI is the main SBI diagnosis, the introduction of PCT does not seem to have had a significant clinical impact in our hospital.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Parada E, Calavia O, Durán-Ballén M, Vasquez A, Ayats R, Ferré N. Valoración del uso de la procalcitonina en el lactante febril hospitalizado. An Pediatr (Barc). 2016;84:278–285.