Type 1 diabetes (T1D) is a hyperglycaemic syndrome resulting from the autoimmune destruction of the pancreatic islet beta cells. After diagnosis and treatment initiation, approximately 50% of patients go through what is known as a partial clinical remission phase,1 of variable duration and characterised by partial recovery of function of beta cells and therefore an increased capacity to produce insulin. The longer this phase lasts, the better the short- and long-term prognosis of diabetes, with a decreased risk of severe hyperglycaemia and chronic complications, improved lipid control and an easier control of diabetes in the future. Prolonging this “honeymoon” phase requires good metabolic control, for which an excellent technological tool is currently available that measures interstitial glucose levels, continuous glucose monitoring (CGM), that should be used in every paediatric patient from the moment diabetes is diagnosed. Positive results have also been reported with the use of monoclonal antibodies such as teplizumab, associated with delayed diagnosis of T1D and prolongation of the honeymoon phase.
Without question, the greatest advances in the treatment of T1D made in recent times involve the use of technology combined with ongoing structured/standardised diabetes education, which is essential in improving disease control, adherence to treatment and patient quality of life.2 Despite all these advances, the objectives set for the paediatric population by international societies (American Diabetes Association [ADA], International Society for Pediatric and Adolescent Diabetes [ISPAD]) are not being met, and therefore other approaches need to be explored to improve adherence to treatment.
It is clear that use of the novel rapid-acting insulins (Fiasp®, approved by the European Union for use in children aged more than 1 year in September 2019, and Lyumjev™, authorised in the United States in June 2020) currently available for both multiple daily injection (MDI) therapy and insulin pumps can improve postprandial glycaemic control. However, the main advances made in recent decades involve the use of insulin pumps,3 CGM systems and the combination of both, along with algorithms for automated insulin delivery and automatic insulin delivery suspension, already in use in clinical practice. In addition, wireless download of data to specific platforms, mostly through the cloud, allows sharing patient information with the caregivers (parents, teachers, diabetes specialists) in real time, allowing for more precise adjustments of treatment in everyday life.
Flash glucose monitoring systems, in which data are only shown when the sensor is scanned, and CGM systems provide information of glucose levels at 5-minute intervals and on glucose level trends. These systems have improved considerably in recent years, in both accuracy and precision, and some are factory-calibrated. At present, the data provided by these systems is precise enough to be used instead of capillary glucose measurements to guide clinical decision-making save in special circumstances. Another important advantage offered by technology is that devices are equipped with alarms and alerts to signal the risk of hyperglycaemia or hypoglycaemia, remind the patient of undelivered boluses or warn of pump blockages (Fig. 1).
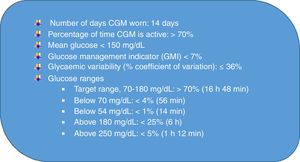
With the development of CGM, we moved from an era when glycaemic control was based on glycated haemoglobin levels to one in which it is based on CGM parameters, the “time-in-range” era.4Fig. 2 presents the international targets, to which glycaemic variability is added through a coefficient of variation, with a percentage of 36% or less used to define stable diabetes.
International continuous glucose monitoring (CGM)-based targets for glycaemic control (adapted from Battelino et al4).
The combination of CGM data downloading to the pump and subsequent interpretation with specific algorithms (sensor-augmented insulin pumps [SAPs]) decreases the risk of hypoglycaemia (predictive low-glucose suspend systems, such as the Medtronic MiniMed 640 G or the Tandem Basal-IQ).
At present, automated insulin delivery systems not only adjust insulin delivery to changes in blood glucose, but also provide automatic corrective bolus delivery,5 a type of system known as hybrid closed-loop, as the user is still required to program insulin boluses for meals and make adjustments to the dose for exercise (Medtronic MiniMed 780 G, which received CE Mark approval in June 2020, and Tandem t:slim X2-Control IQ, approved in the United States in 2020 for use in individuals aged more than 6 years).
In the near future, bihormonal closed-loop systems will be available to automatically deliver both insulin and glucagon as needed (iLet, Beta Bionics). Implementation of bihormonal systems was delayed because they required a form of glucagon that would remain stable in solution, which has finally become available (Dasiglucagon). Also, research on new formulations of glucagon led to the development of a powder form of glucagon for nasal delivery (BAQSIMI™, Lilly), approved by the European Union in 2020 for use in children aged more than 4 years, and a solution contained in pre-filled syringes for self-injection available in the United States (Gvoke™ HypoPen) for individuals aged more than 2 years. These new dosage forms constitute advances in the treatment of severe hypoglycaemic episodes, which continue to be a significant problem.
Since most patients are managed with MDI, we ought to highlight the advances offered by the use of technology in smart insulin pens, which provide data on administered doses, times of injection and insulin temperature. Some of these devices work with bolus calculator apps that help calculate doses for meals or blood glucose corrections taking into account the amount of insulin that is still active.
Last of all, we ought to mention the technological advances that have allowed the introduction of telemedicine services and made data sharing possible. Diabetes may be the chronic disease that could benefit most from telemedicine consultations, as demonstrated during the coronavirus 2019 pandemic. Telemedicine visits are not only useful for glycaemic control, but also for health education, psychological support and closer contact between the patient and the diabetes management team, saving time, eliminating travel, decreasing the hours of missed school and work and preventing contagion during outbreaks and pandemics.
To conclude, it is fair to say that while we wait for current research efforts that are mainly focused on T1D prevention, the protection of insulin-producing cells to slow down disease progression and cell-replacement therapy to bear fruit, advances in technology and new insulin formulations will unquestionably continue to contribute to the improvement and optimization of glycaemic control in individuals with T1D.
FundingThis study was not supported by any source of funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Barrio Castellanos R. Avances en el tratamiento de la diabetes tipo 1 pediátrica. An Pediatr (Barc). 2021;94:65–67.