The performing of complementary tests in infectious processes can increase the diagnostic precision, the adequacy of treatments, as well as determining the epidemiology and pattern of bacterial resistance of the community. The Infectious Pathology Group of the Spanish Association of Primary Care Paediatrics (GPI-AEPap) has designed this study in order to determine the availability of complementary tests (CT) for paediatricians working in Primary Care of the public health system as well as their results.
Material and methodsObservational cross-sectional descriptive national study was carried out using a voluntary self-report questionnaire distributed online to all AEPap members and to the subscribers of the PEDIAP distribution list between the months of April and May 2017.
ResultsA total of 517 responses were obtained. An analysis was made of the data from the professional environment, as well as those related to the request for basic supplementary tests (blood count, biochemistry, and routine urine analysis), the use of Rapid Antigen Detection Test for group A Streptococcus, bacterial cultures, serology, diagnostic tests for pertussis and tuberculosis (Mantoux), as well as imaging tests.
ConclusionsThere is variability between Autonomous Communities and healthcare areas. Areas for improvement were found in the accessibility to different CT, collection time and sending of samples, delay in receiving results, as well as waiting times for non-urgent imaging tests. These affect the intervention and resolution capacity of the primary care paediatrician.
En los procesos infecciosos la realización de pruebas complementarias puede aumentar la precisión diagnóstica, la adecuación de los tratamientos, así como dar a conocer la epidemiología y patrón de resistencias bacterianas de la comunidad. El Grupo de Patología Infecciosa de la Asociación Española de Pediatría de Atención Primaria (GPI-AEPap) diseña este estudio para conocer la accesibilidad a pruebas complementarias (PC) y sus resultados que tienen los pediatras que trabajan en atención primaria en el ámbito de la salud pública.
Material y métodosEstudio observacional transversal descriptivo de ámbito nacional, a través de una encuesta de cumplimentación voluntaria, distribuida on line a todos los socios de AEPap y a los suscriptores de la lista de distribución PEDIAP entre los meses de abril y mayo de 2017.
Resultados517 respuestas. Se analizan datos del entorno profesional así como los referidos a la solicitud de pruebas complementarias básicas (hemograma, bioquímica, sistemático de orina), utilización de Test Rápido Detección de Antígeno para Streptococo grupo A (TRDA), sobre cultivos bacterianos, serologías, pruebas diagnósticas de tosferina y tuberculosis (mantoux) y pruebas de imagen.
ConclusionesHay variabilidad entre CCAA y áreas asistenciales. Se detectan claras áreas de mejora en la accesibilidad a diferentes PC, tiempo de recogida y envío de muestras, demora en recepción de resultados así como en tiempos de espera para pruebas de imagen no urgentes. Esto interfiere en la capacidad de intervención y resolución del pediatra de atención primaria.
Most paediatric diseases are managed and resolved at the primary care (PC) level without need of diagnostic tests (DTs). However, in some infectious diseases, access to DTs can increase the accuracy of diagnosis, help determine appropriate treatment and make earlier and appropriate referrals.1 Barriers to access to DTs in PC paediatric clinics (ordering test, performance of test, delivery of results, turnaround times) can affect or hinder clinical practice, rational use of antibiotics, performance of research studies and knowledge of local epidemiology and increase the frequency of referral to hospitals, among other aspects.
Primary care paediatric services are located in different settings: urban, semi-urban and rural, in which the accessibility of laboratory, microbiology and radiology services may vary based on distance, hours of operation and frequency of sample collection and the referral or submission pathways established in the different health care areas. Based on previous studies, only 29% of providers can order all laboratory or imaging tests without restrictions from the PC setting, and only 18% have access to all tests following a given protocol.2,3
ObjectiveThe objective of the study, promoted by the Group on Infectious Diseases (GID) of the Asociación Española de Pediatría de Atención Primaria (Spanish Association of Primary Care Paediatrics, AEPap),4 was to determine the access of primary care paediatricians (PCPs) working in the public health system to ordering and performing tests for diagnosis of infectious diseases in their health care centres, their access to tests results and the associated turnaround times.
Material and methodsWe conducted a cross-sectional, observational descriptive nationwide study in PCPs employed in the public health system through completion of a questionnaire, on a voluntary and unpaid basis, distributed electronically between April and May 2017 (available at https://goo.gl/forms/VYNoqiuQhojACv2Z2). We recruited participants from the list of members of the AEPap, a professional association of PC paediatricians, and also sent the questionnaire to subscribers of the PEDIAP mailing list,5 created in 2000 as a platform for professionals to network and discuss subjects of interest in the field of PC paediatrics, who amount to approximately 1000 and are very representative of the PC paediatrician collective. We specifically requested that only paediatricians involved in PC, exclusively or in combination with other activities, respond to the survey, clarifying that responses should refer solely to the primary care setting. In the 2 months that the questionnaire was available for completion, we sent 2 reminders.
We collected data on the characteristics of the respondents, including age, sex, work setting, caseload, availability of paediatric nursing staff and the degree of access to a hospital. When it came to diagnostic tests, we grouped them by type of disease: acute pharyngotonsillitis (AP), urinalysis (urine strip test, sediment and culture); other bacterial cultures and serological tests, diagnostic tests used in pertussis and tuberculosis and imaging tests.
We expressed and tabulated qualitative data as percentages with the corresponding 95% confidence intervals (CIs). We expressed quantitative data as mean and standard deviation. We performed a univariate analysis with calculation of odds ratios (ORs) to make comparisons, using the χ2 square test and defining statistical significance as a p-value of 0.05 or less.
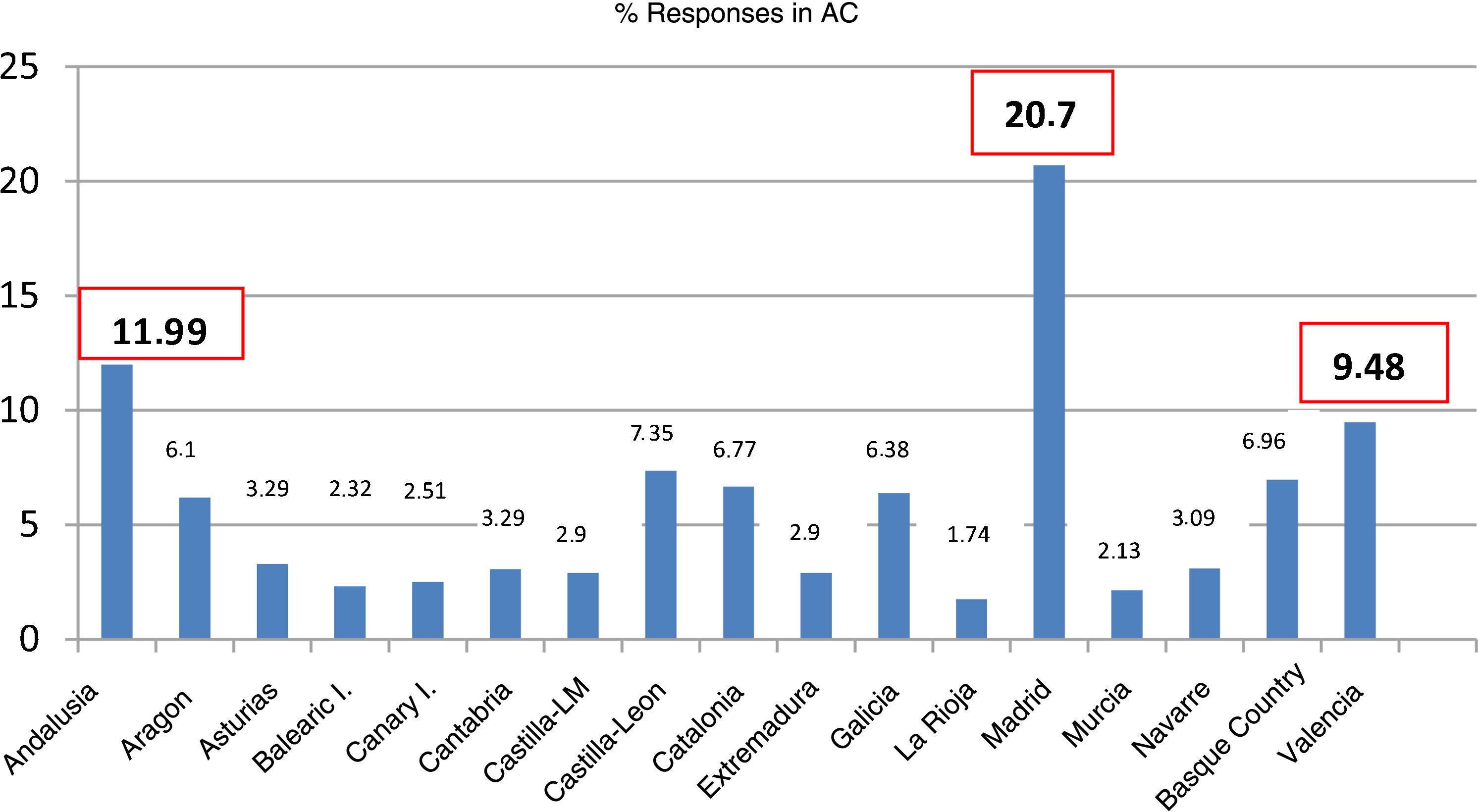
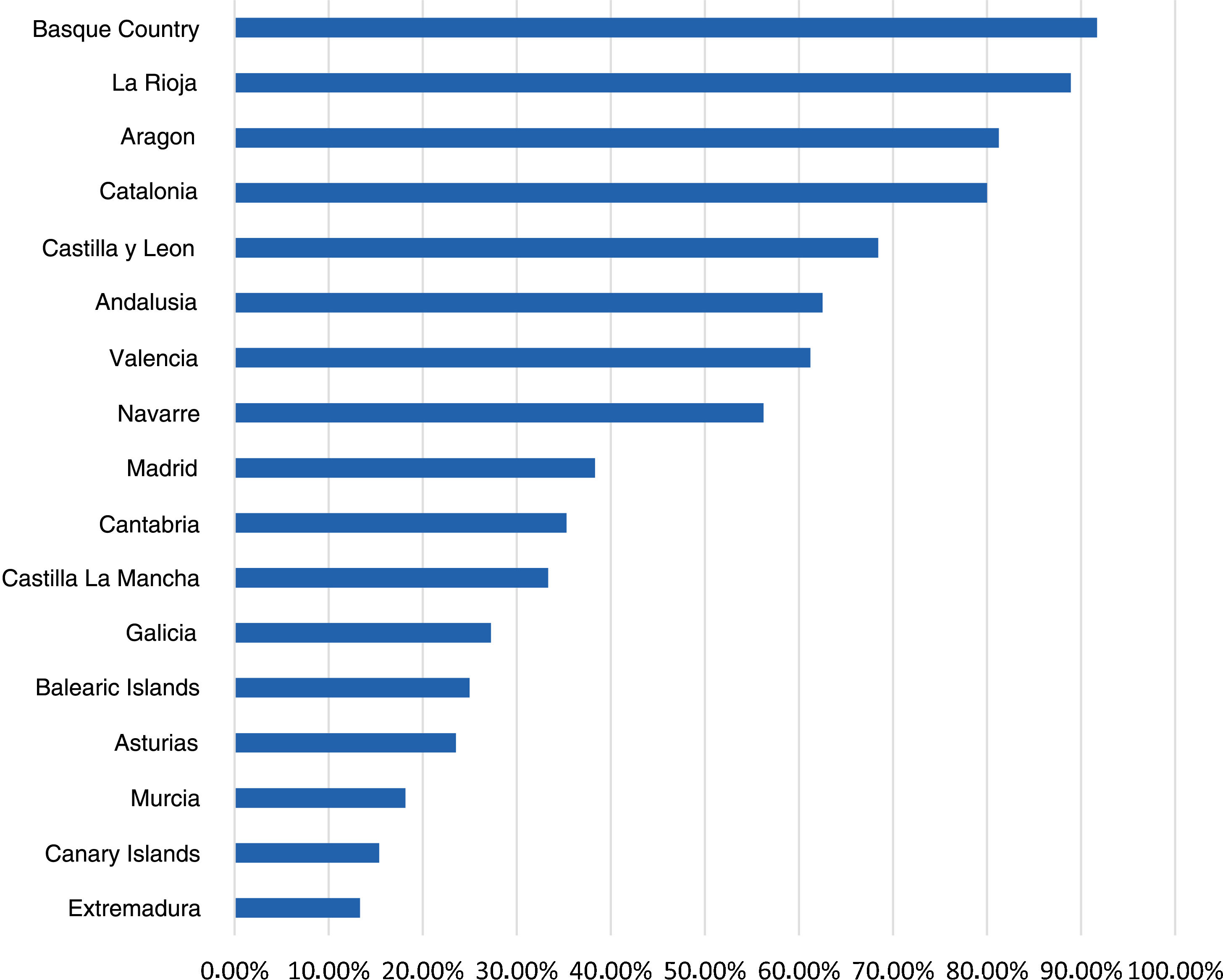
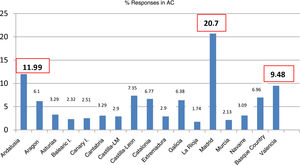
ResultsCharacteristics of participating paediatricians and sitesWe received responses from 517 PCPs (Table 1), with the greatest proportions corresponding to the regions of the Community of Madrid (20.7%), Andalusia (11.9%) and the Valencian Community (9.4%) (Fig. 1).
Paediatricians that responded to the survey by autonomous community (AC).
| AC | Absolute frequency | % over total in AC |
|---|---|---|
| Andalusia | 62 | 11.99 |
| Aragon | 32 | 6.19 |
| Principality of Asturias | 17 | 3.29 |
| Balearic Islands | 12 | 2.32 |
| Canary Islands | 13 | 2.51 |
| Cantabria | 17 | 3.29 |
| Castilla and Leon | 38 | 7.35 |
| Castilla-La Mancha | 15 | 2.90 |
| Catalonia | 35 | 6.77 |
| Valencian Community | 49 | 9.48 |
| Extremadura | 15 | 2.90 |
| Galicia | 33 | 6.38 |
| Community of Madrid | 107 | 20.70 |
| Region of Murcia | 11 | 2.13 |
| Navarre | 16 | 3.09 |
| Basque Country | 36 | 6.96 |
| La Rioja | 9 | 1.74 |
| Ceuta and Melilla | 0 | 0 |
| Total | 517 | 100 |
Of all respondents, 82.6% were female (95% CI, 79%-86%) and 71.4% were aged 45–65 years. The majority (79.8%) worked in towns with populations of 10 000 inhabitants or greater, and 58.6% had caseloads of 1000–1500 patients. Fifty-nine percent worked the morning shift and 33.8% mixed shifts. Fifty-five percent reported seeing between 20 and 30 children a day. Nearly ¾ of paediatricians had access to a paediatric nurse all or most of the time. The same percentage considered that the staff in charge of collecting samples were poorly qualified for the purpose. As for access to a hospital, 57% reported it was totally or very available.
We found that 32.9% of paediatricians could not collect samples every day. The time window for sample collection in the days it was performed was of 1 h for 46.7% of respondents and of 1–2 hours for 86%. In addition, 44.1% reported that it was difficult or very difficult for them to submit samples outside the established schedule. As for the means to access or receive laboratory test results, 56% accessed them via the laboratory website, 31.9% reported that results were uploaded to the electronic health record database and 10% received printed test results.
Basic diagnostic testsSixty-nine percent of paediatricians ordered at least one complete blood count and 1 chemistry panel each week. Results were available in 24 h to 75.1% for the complete blood count and to 70.2% for the chemistry panel. Ninety-nine percent of respondents used urinalysis regularly. Seventy-three percent obtained the results within 24 h. Also, 89.9% ordered C-reactive protein tests (only 4.9% by micromethod). Seventy percent obtained the results in 24 h.
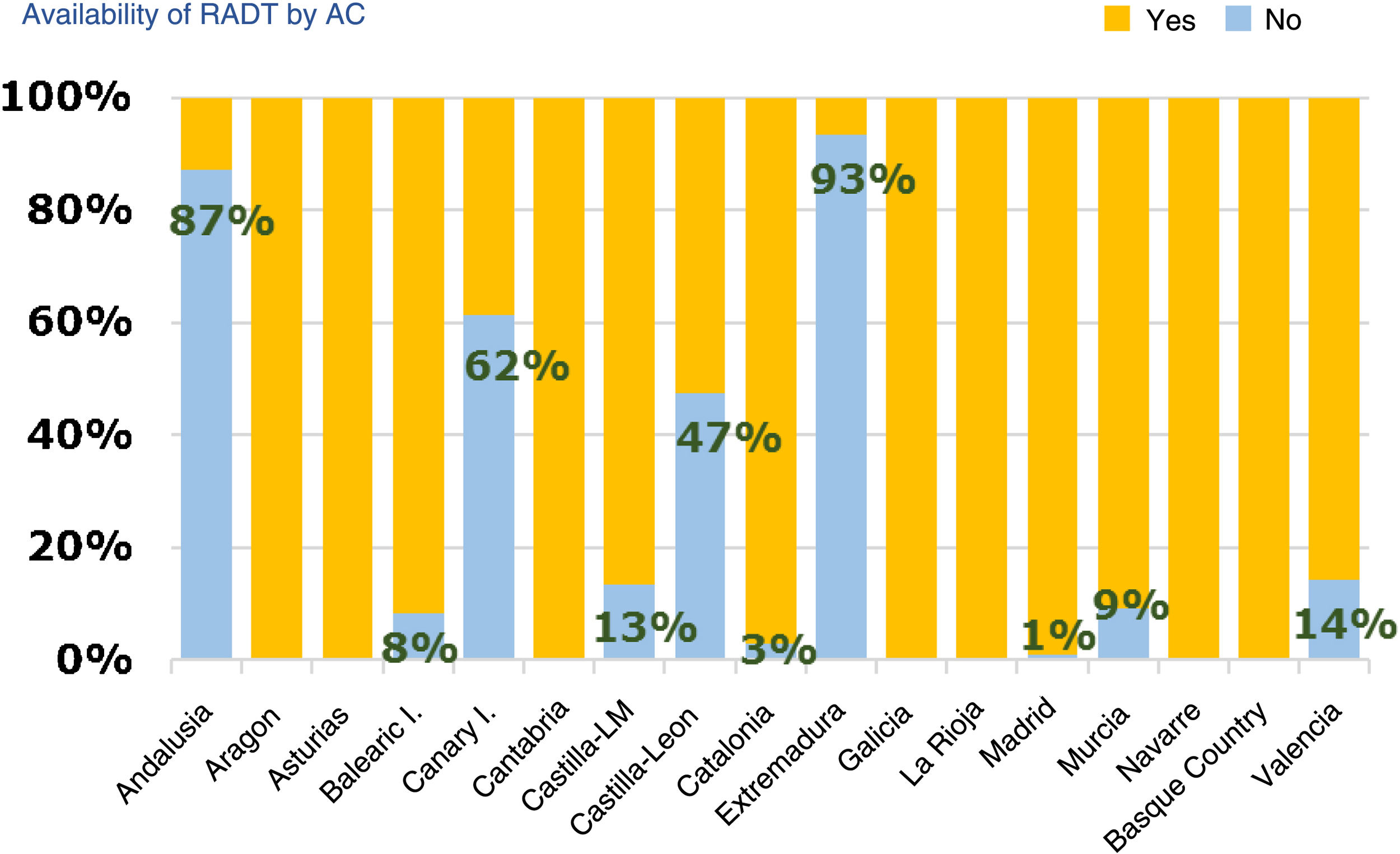
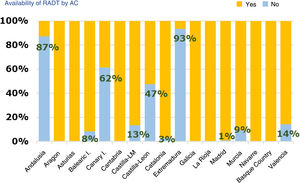
Diagnostic tests for management of pharyngotonsillitisSeventy-nine percent of respondents had rapid antigen detection tests (RADTs) done (95% CI, 76%-83%). Of those that did not have access to RADTs, 79% ordered cultures (95% CI, 72%-87%), while of those that had access to RADTs, 82% ordered cultures occasionally (95% CI, 78%-82%). Of those respondents that did not use RADTs, 95% reported it was due to lack of access. Andalusia, Extremadura, the Canary Islands and Castilla and Leon were the region where these tests were least available (Fig. 2). Seventy-eight percent of providers (95% CI, 74%-82%) reported performing RADTs themselves, especially providers aged less than 45 years (77%) compared to older providers (67%) (OR, 1.67; 95% CI, 1.07–2.60; P = .012). Sixty-seven percent of respondents that managed more than 30 patients a day did these tests themselves, compared to 65% of those managing fewer than 30 patients a day.
The criteria based on which providers ordered RADTs or cultures were, in order of decreasing frequency: a score of 3–4 in the Centor scale (43%), the provider’s own professional experience (33%), stigmata compatible with infection by Streptococcus pyogenes (26%) or fulfilment of more than 2 criteria in the McIsaac score (18%). Among those with access to RADTs, 23% did fewer than 1 a week, 45% did 1–3 a week and 32% did 4 or more a week. Cultures were ordered less frequently, for in the group of respondents that had no access to rapid testing, 62% ordered fewer than 1 culture a week, 36% ordered 1–3 cultures a week, and 2% ordered 4 or more. Fifty percent of providers had to wait 4 or more days to receive the results of throat cultures.
Urine sample collection methods and urine testsIn children without bladder control, most urine samples were obtained with a collection bag (88%) or were midstream clean catch specimens (70%). Only 18% of respondents reported using catheter samples, and the reasons cited to not use this approach were lack of training on the technique, lack of supplies or lack of time. Primary care paediatricians were generally responsible for providing instruction on how to collect urine samples (more than 90%) and up to 77% performed urine strip tests themselves.
Seventy-three percent of providers received the results of urinalysis within 24 h and 45% had to wait 4 or more days for the results of urine culture.
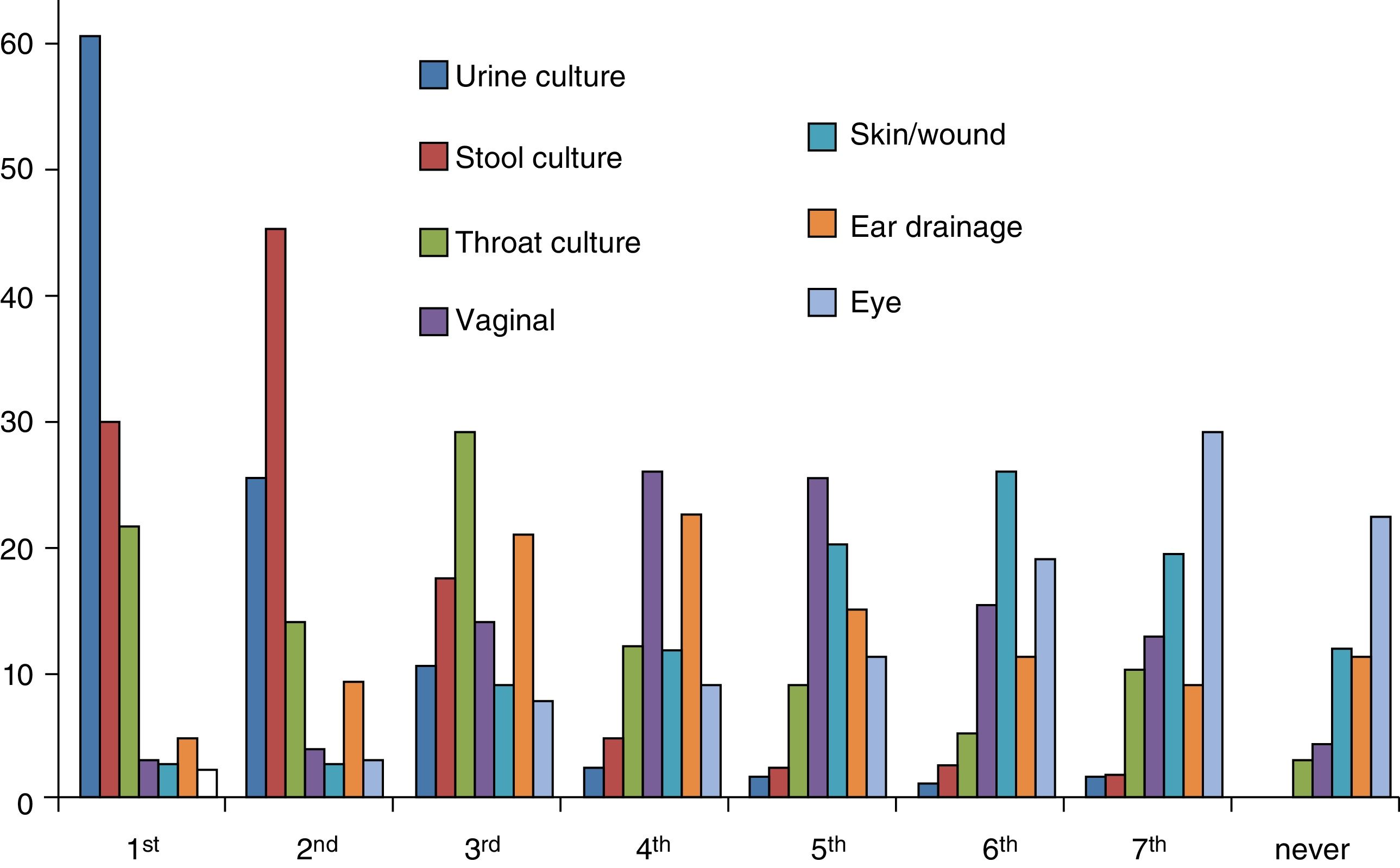
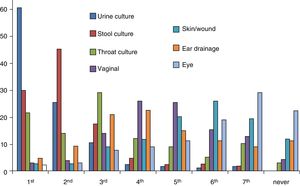
Bacterial cultureWe collected data on urine, stool, throat, ear drainage, vaginal, eye and skin/wound cultures. Urine cultures were the type of culture ordered most frequently by 60.2% of paediatricians, stool cultures the second most frequently ordered by 45%, and throat cultures the third most frequently ordered by 28.7% (Fig. 3). In addition, 31.5% of paediatricians ordered at least 1 throat culture a week and 61.3% at least 1 stool culture a week. Also, 66.7% of paediatricians ordered urine cultures (collection bag sample in 50%, catheter sample in 2.2% and referral to secondary care facility for obtention of catheter sample in 14.5%), and 29.8% reported ordering other types of culture. When it came to the turnaround time, it took four days or longer to receive the results of urine cultures for 44% of paediatricians, the results of throat cultures for 50% and the results of all other cultures for 60%.
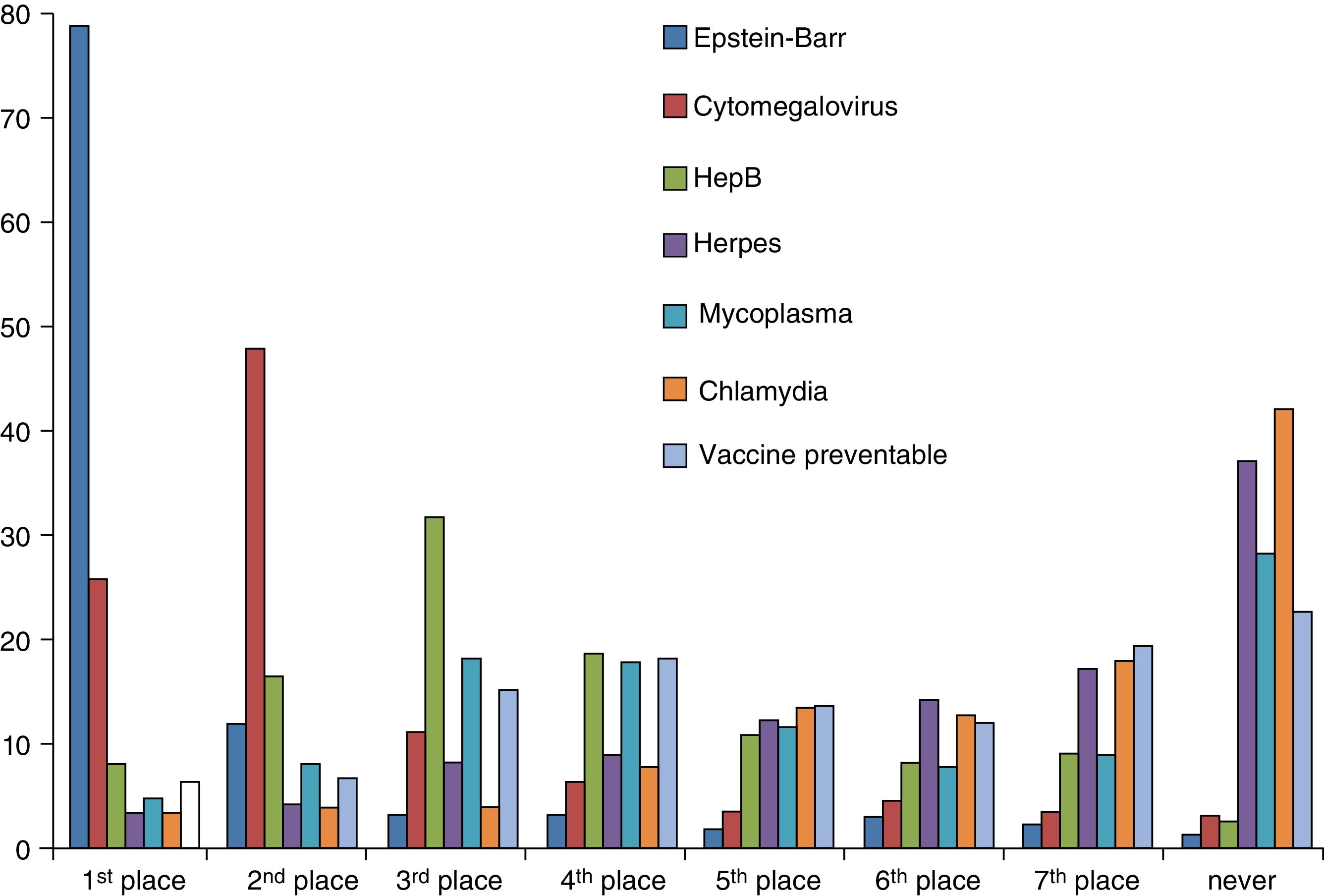
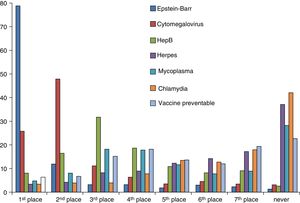
SerologyA total of 78.6% of surveyed paediatricians ordered fewer than 1 serological test per week. The serological test ordered most frequently by 78% of respondents was the test for detection of Epstein-Barr virus, followed by cytomegalovirus and hepatitis B tests. Serological tests for Mycoplasma, Chlamydia and herpes were never ordered by 27.7%, 41.4% and 36.4% of paediatricians, respectively (Fig. 4). The percentage of respondents that reported turnaround times of 7 days or longer was 30% for Epstein-Barr virus tests, 32% for cytomegalovirus and hepatitis B tests, and 43% to 44% for tests for detection of Mycoplasma, Chlamydia, herpes and other vaccine-preventable diseases.
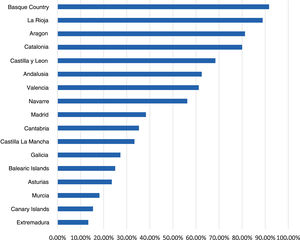
Diagnostic tests for pertussisThe data obtained on the use of DTs for pertussis revealed considerable differences between autonomous communities, with these tests used least frequently in Extremadura and the Canary Islands (13.3% and 15.4%) and most frequently in the Basque Country and La Rioja (91.7% and 88.9%, respectively) (Fig. 5). Fifty-three percent of respondents reported using some form of test for diagnosis of pertussis: polymerase chain reaction (PCR), serology and/or culture. Of those that did not, 77.5% explained it was due to a lack of availability. The technique most frequently reported as available was PCR (87%), although 62% of respondents had to refer the patient for collection of the sample. Respondents aged 45–65 years used these DTs more frequently compared to younger respondents (55% vs 48%). In this survey, 92.7% of paediatricians reported ordering fewer than 1 PCR test a week for detection of pertussis. Only 2% received the results the same day, and 23% in 1–2 days.
Mantoux testThis test was used by 90% of respondents. The only reason cited for not using it was a lack of availability. Respondents personally interpreted the test in 68.1% of cases, mainly in the 45- to 65-year age group. Forty-nine percent of paediatricians reported that shortages in tuberculin antigen affected the number of tests they ordered. We found that 92.7% ordered fewer than 1 Mantoux test a week.
Imaging testsImaging tests (plain radiography and ultrasound) were accessible to 97% to 100% of respondents. In most instances they were performed in a hospital setting (91.6%). Nearly 100% of samples reported that the performance of the tests considered urgent by the PCP for which patients were referred to hospital depended on the decision of the emergency care physician or on-call radiologist.
Two percent of respondents personally performed ultrasound examinations at their sites.
Eighty-two percent of paediatricians accessed test results through the internet, while 17% had tests results automatically uploaded to the electronic health record database. The delay in the appointments for elective plain X-rays was of 7 days or greater in 47% of cases, and only 11% of respondents reported appointments being available within 2 days. The delay in obtaining appointments for elective ultrasound examinations was of more than 1 month in 60% of cases and more than 2 months in 27.5%.
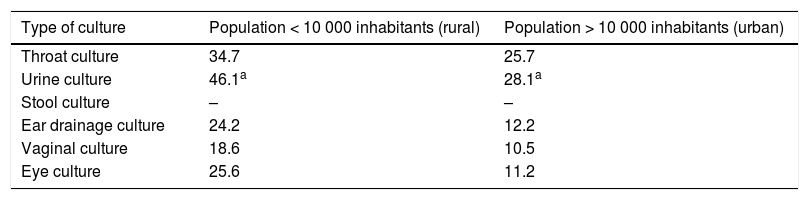
Turnaround time and availability of test results in rural and urban settings (municipalities with < 10 000 inhabitants vs > 10 000 inhabitants)The results of complete blood counts were available on day 1 for 34% of paediatricians in urban settings vs 38.4% of those in rural settings, a difference that was not statistically significant (OR, 0.83; 95% CI, 0,52 to 1,31: P = .4122). In urban settings, 23.1% of paediatricians received blood chemistry test results within 24 h, compared to 30.6% of respondents in rural settings, although the difference was not statistically significant (OR, 0.68; 95% CI, 0.42–1.12; P = .1258) (Table 2).
Availability of diagnostic test results by type of setting, expressed as the percentage of paediatricians. Differences were not statistically significant difference (χ2).
| Diagnostic test | Population < 10 000 inhabitants (rural) | Population > 10 000 inhabitants (urban) | ||
|---|---|---|---|---|
| 1 day | 48 h | 1 day | 48 h | |
| Complete blood count | 38.4 | 70.7 | 34 | 76.6 |
| Chemistry panel | 30.6 | 69.4 | 23.1 | 71 |
When it came to the availability of test results within 3 days, we did not find statistically significant differences between providers in urban versus rural settings, save for urine culture, which had a significantly shorter turnaround time in towns with less than 10 000 inhabitants (46.1% versus 28.1% received results within 3 days) (OR, 2.19; 95% CI, 1.40–3.42; P = .0005) (Table 3).
Availability of culture results within 3 days by type of setting, expressed as the percentage of paediatricians. * Statistically significant difference (χ2).
Most infectious diseases managed by PCPs do not require performance of diagnostic tests, but the availability of the latter is relevant when it comes to the decision whether to prescribe an antibiotic agent, to obtain data on antimicrobial resistance in the community on which to base recommendations for empiric antibiotherapy and to identify and control the spread of infections. Diagnostic tests can also allow earlier diagnosis and initiation of treatment.
Based on previous data, it appears that the number of days in which samples can be collected and submitted remains stable (2012 PAPE survey)3: 71% of respondents could collect and submit blood samples Monday through Friday.
Only 31.9% had laboratory test results automatically uploaded to patient health records, a proportion that should be increased to integrate information and to facilitate and expedite access to test results by primary care providers.
Acute pharyngotonsillitis is one of the most common childhood diseases, and while there are guidelines for the prescription of antibiotherapy, there is still evidence of frequent and inappropriate prescription for treatment of this disease.6,7 The aetiology is usually viral, and the most frequent bacterial causative agent is Streptococcus pyogenes or group A beta-haemolytic streptococcus ( (GABHS). Even in patients with the highest scores in the most widely used clinical rules, the probability of APT caused by S. pyogenes does not exceed 60% to 64%, so the suspected diagnosis should be confirmed by microbiological methods. Although throat culture is the gold standard, a high percentage of paediatricians did not use it routinely in their practices due to the long turnaround times. There is evidence of the usefulness of RADTs,8 possibly exceeding that of culture, in reducing antibiotic prescription in the context of APT,9 and therefore these tests should be used by every provider. At present, while they are widely used (79% of respondents reported ordering these tests), the reason given by 95% of those that did not use rapid tests was that they were not available, with substantial differences between autonomous communities in their availability.
Collection of samples for bacterial culture has proven useful10 to improve rational prescription of antibiotics at the time of initiation of treatment and for its adjustment, suspension or replacement by a different agent once results are available. Restrictions in the times that samples can be collected or submitted at the primary care level (1/3 of respondents could not collect samples on every weekday, and the time window for submission was 1 h for nearly half of providers), and delays in obtaining the results (≥ 4 days for 44% of respondents for urine cultures and for 50% of respondents for throat cultures) are a significant barrier to rational use of antibiotics. This may entail referral of patients to hospital-based services (which are difficult to access for 43% of respondents), in which case not only may patients be inconvenienced by having to travel to the site, but the performance of the tests requested by the PCP depends on decisions made by a different professional, which thwarts the considerable capacity of primary care services for resolving problems presenting at that level.
These limitations particularly affect the diagnosis and treatment of urinary tract infections (UTIs), in which confirmation of the diagnosis, identification of the causative agent and establishment of antimicrobial resistance patterns are essential to provide appropriate treatment to the patient and obtain information on the local epidemiology. The diagnosis of UTIs continues to be a challenge, especially in children without bladder control, due to the nonspecificity of the symptoms and the difficulty of obtaining a valid sample. In these children, most samples are obtained with a collection bag or through a midstream catch, methods associated with a high frequency of contamination.11 Thus, positive culture results these samples require confirmation in catheter urine sample, and therefore referral of the patient for performance of this technique (only 18% performed insertion of urinary catheters at the health care centre). A recently published guideline on the management of UTIs excludes the option of performing cultures of samples obtained with a collection bag,12 adherence to which would entail the referral of nearly all patients with suspected UTI.
On the subject of the turnaround time, a study conducted in Andalusia in 200913 found that 3% of PCPs received the results of complete blood counts within 24 h compared to 83.3% of hospital-based paediatricians (HPs). A similar pattern was found for X-ray results, received in 1 day by 68.8% of paediatricians in primary care compared to 85.7% of hospital paediatricians. The differences in the availability of urine culture results at 48 h were even more marked, corresponding to 4.5% of PCPs compared to 45.2% of HPs, with significantly longer turnaround times at the primary care level.
In spite of these factors, the collection of samples for culture in PC is relevant for the purpose of establishing the epidemiology of community-acquired infections, the causative agents involved and their drug resistance profile.14,15
Access to DTs and a short turnaround time are particularly relevant in diseases like tuberculosis (TB) and pertussis, in which early diagnosis is key to initiate treatment and control outbreaks. The Mantoux test is the gold standard for initial diagnosis and contact screening of TB.16,17 It is usually performed in the primary care setting, although our findings show the effects of a temporary shortage of tuberculin antigen. As for pertussis, since neither the infection nor vaccination confer lifelong immunity and the presentation is often atypical, a high level of suspicion is required for early detection of cases and prevention of transmission,18 while a certain diagnosis is important to prevent the use of antibiotics in unconfirmed cases. At present, PCR is considered the gold standard19 and it was available to 87% of respondents, although 62% of PCPs needed to refer the patient for sample collection, with differences between autonomous communities. Once again, the long turnaround times (only 2% receive the results in the day and 23% in 1–2 days) pose a barrier to early diagnosis, treatment and initiation of antibiotic prophylaxis.
In general, serologic tests were ordered infrequently at the primary care level, perhaps because they tend to be ordered to guide clinical decision-making and the delays in obtaining the results undermine this purpose.
The results involving imaging tests were pretty homogeneous across autonomous communities. Access to these tests was optimal, although the delays in getting an appointment for elective ultrasound examinations could be improved.
When it came to ultrasound examinations, primary care paediatricians had little autonomy, as the actual performance of requested tests was left to the judgment of hospital-based providers.
There is evidence that point-of-care ultrasound performed by clinicians, including primary care paediatricians,20 may be useful for diagnosis of different diseases.21,22 However, few paediatricians order or perform point-of-care ultrasound examinations at the primary care level due to their excessive workloads and the lack of sonographers on staff.23
The task of collecting samples for testing falls within the nursing scope of practice, but we found a considerable involvement of paediatricians, independently of the size of their caseloads. This may be explained by the frequent lack of paediatric nurses in primary care settings, as many operate under a community health nursing model in which nurses are shared by paediatric and family medicine teams, have greater workloads and are less familiar with paediatric care, and PCPs end up performing these tasks.24
When we compared the turnaround of results in sites serving populations greater and smaller than 10 000 inhabitants, we found that the results were not delayed in smaller towns.
There are no studies in the literature providing data on the overall access of primary care paediatricians to the ordering and/or performance of diagnostic tests. It would be interesting to replicate this study in the future to analyse how the availability of diagnostic tests changes over time.
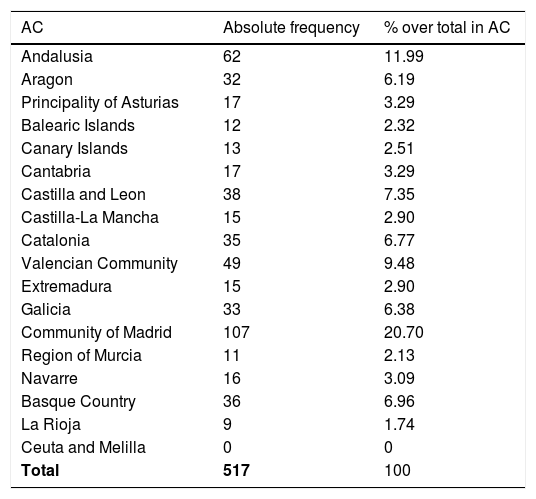
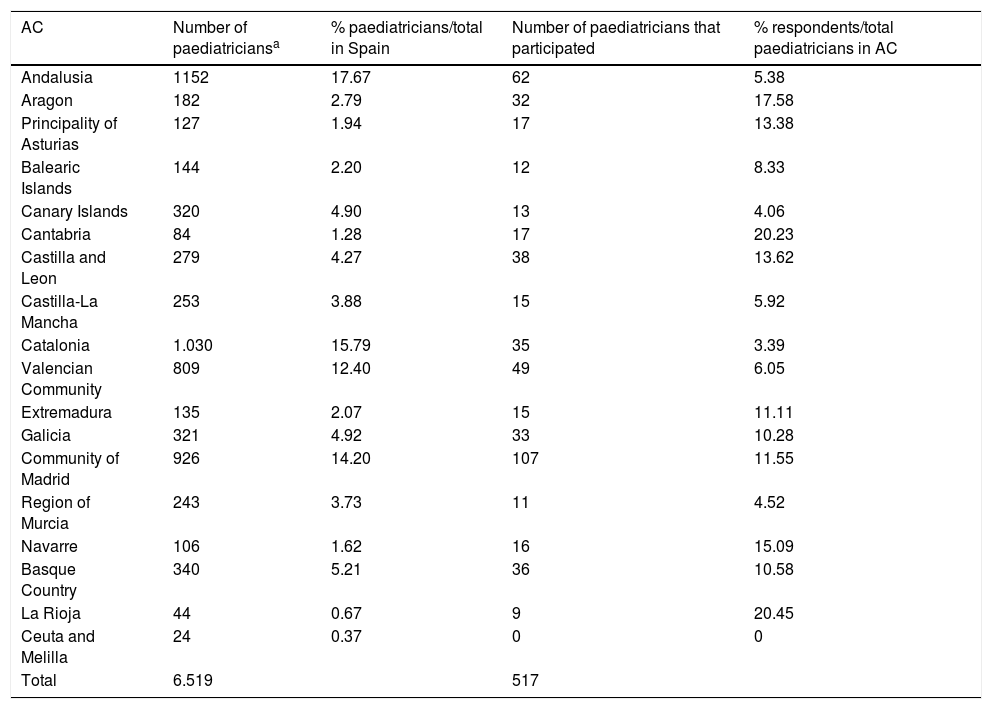
Limitations of the studyThere are limitations to our study. Given the observational design and that the survey was conducted in a particular period of time, the data obtained may be subject to the biases intrinsic in this type of study. Since participation was voluntary, it may have been affected by the characteristics of the work setting and the availability of tests. There is also a risk of selection bias through the lack of responses from paediatricians who are not members of the AEPap and/or not subscribed to the PEDIAP mailing list. The response rate in our survey was 7.93% (95% CI, 7.27%-8.59%) based on the total number of primary care paediatrician positions in the Spanish health care systems, 6519 according to 2017 data published in the Statistics Portal of the Ministry of Health, Social Welfare and Equality. Although participation did not suffice for the sample to be representative of every autonomous community in Spain (Table 4), it was sufficient at the national level, so our findings offer a good approximation to the actual availability of diagnostic tools in each of the regions. Furthermore, since infectious diseases exhibit significant seasonal patterns, there is a risk of recall bias depending on the time of the year that the questionnaire is completed.
Table comparing the number of paediatricians to the number of responses per autonomous community (AC) in Spain.
| AC | Number of paediatriciansa | % paediatricians/total in Spain | Number of paediatricians that participated | % respondents/total paediatricians in AC |
|---|---|---|---|---|
| Andalusia | 1152 | 17.67 | 62 | 5.38 |
| Aragon | 182 | 2.79 | 32 | 17.58 |
| Principality of Asturias | 127 | 1.94 | 17 | 13.38 |
| Balearic Islands | 144 | 2.20 | 12 | 8.33 |
| Canary Islands | 320 | 4.90 | 13 | 4.06 |
| Cantabria | 84 | 1.28 | 17 | 20.23 |
| Castilla and Leon | 279 | 4.27 | 38 | 13.62 |
| Castilla-La Mancha | 253 | 3.88 | 15 | 5.92 |
| Catalonia | 1.030 | 15.79 | 35 | 3.39 |
| Valencian Community | 809 | 12.40 | 49 | 6.05 |
| Extremadura | 135 | 2.07 | 15 | 11.11 |
| Galicia | 321 | 4.92 | 33 | 10.28 |
| Community of Madrid | 926 | 14.20 | 107 | 11.55 |
| Region of Murcia | 243 | 3.73 | 11 | 4.52 |
| Navarre | 106 | 1.62 | 16 | 15.09 |
| Basque Country | 340 | 5.21 | 36 | 10.58 |
| La Rioja | 44 | 0.67 | 9 | 20.45 |
| Ceuta and Melilla | 24 | 0.37 | 0 | 0 |
| Total | 6.519 | 517 |
We found differences between autonomous communities and care settings. There are clear opportunities for improvement in the access to different DTs, the time elapsed to collection and submission of samples, the test turnaround time and the wait times for performance of elective imaging tests. This interferes with the ability of primary care paediatricians to manage and resolve presenting complaints.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all our colleagues who generously collaborated in this study with no remuneration by completing the questionnaire, the responses to which are the basis of this study.
Please cite this article as: Martin-Peinador Y, Albañil-Ballesteros MR, García-Vera C, Jimenez-Alés R, Muñoz-Hiraldo E, Martínez-Chamorro MJ et al. Acceso a pruebas complementarias para el diagnóstico de enfermedades infecciosas en las consultas de pediatría de atención primaria. Estudio beenis. An Pediatr (Barc). 2021;94:82–91.