To compare plasma glucose levels and incidence of hyperglycaemia in the post-operative period after general surgery using fluids with different glucose.
MethodologyA randomised, open-label, non-blind, clinical trial was conducted on patients admitted to Paediatric Intensive Care Unit after elective surgery. The inclusion criteria were from 6 months to 14 years of age, with a weight greater than 6kg, onset glucose level >60mg/dL, and a signed informed consent, with no oral intake and maintenance intravenous fluid therapy using fluids with 3.3% or 5% glucose. Plasma glucose levels were measured before surgery, on admission, and 8, 24, and 48h, with the mean glucose levels and incidence of hyperglycaemia (glucose level >150mg/dL) in both groups being compared.
ResultsA total of 60 patients received glucose/saline 1/3 (51mEq/L sodium and 33g/L glucose), and 70 glucose/saline 5/0.9% (154mEq/L sodium and 50g/L glucose). Mean glucose levels were higher in the group receiving glucose 5%, with no statistical difference. There was no significant difference in the incidence of hyperglycaemia; 8h: 26% in the 3.3% group vs. 21.3% in the 5% group (P=.63); 24h: 20% vs. 22.7% (P=.8); and 48h: 19% vs. 23.1% (P=.78).
ConclusionsThe use of fluids with 3.3% glucose in the post-operative period of general surgery maintains mean glucose levels in a similar range to that of patients receiving fluids with 5% glucose, with no difference in the incidence of hyperglycaemia.
Comparar los niveles de glucemia e incidencia de hiperglucemia en el postoperatorio de cirugía general usando sueros con diferente concentración de glucosa.
MetodologíaEnsayo clínico aleatorizado, abierto, no ciego, en pacientes no diabéticos, que ingresan en Cuidados Intensivos Pediátricos tras cirugía electiva, de 6 meses a 14 años, peso superior a 6kg, glucemia>60mg/dl y firma de consentimiento informado, manteniéndose a dieta con sueroterapia de mantenimiento intravenosa mediante suero con glucosa al 3,3 o 5%. Se determinan niveles de glucemia preoperatoria, al ingreso, y a las 8, 24 y 48h, comparando los valores medios y la incidencia de hiperglucemia (glucemia >150mg/dl) en ambos grupos.
ResultadosUn total de 60 pacientes recibieron suero glucosalino 1/3 (51mEq/l de sodio y 33g/l de glucosa) y 70 pacientes suero glucosalino 5/0,9% (154mEq/l de sodio y 50g/l de glucosa). La glucemia media fue mayor en el grupo al 5%, sin diferencia estadística. No hubo diferencia en la incidencia de hiperglucemia; 8h: 26% del grupo 3,3% vs. 21,3% del grupo 5% (p=0,63); 24h: 20% vs. 22,7% (p=0,8); 48h: 19% vs. 23,1% (p=0,78).
ConclusionesEn el postoperatorio de cirugía general, el uso de soluciones glucosadas al 3,3% consigue niveles de glucemia similares a los detectados en pacientes que reciben suero con glucosa 5%, con una incidencia de hiperglucemia similar.
Intravenous (IV) fluid therapy is necessary to meet the water, electrolyte and energy requirements of hospitalised patients when oral intake is not possible or indicated. Although there is nearly universal consensus on the use of isotonic fluids for maintenance fluid therapy,1–7 the optimal dextrose concentration for fluid therapy in the immediate postoperative period has yet to be established. Although the recommended dextrose concentration for maintenance fluid therapy has traditionally been 5% (10% in newborns),8,9 there is growing debate that it may be preferable to use lower dextrose concentrations to prevent the development of hyperglycaemia and its potential harmful effects in critical patients. There is evidence in the paediatric literature of an association between hypoglycaemia or hyperglycaemia and increased morbidity and mortality in critical patients, which has also been described in adults. Several possible mechanisms have been proposed to explain this association, such as increased release of proinflammatory cytokines, acute dyslipidaemia, endothelial dysfunction, hypercoagulability or increased glucose toxicity leading to apoptosis and cell death.10,11
Patients and methodsWe performed a single-centre, prospective, open-label phase IV randomised controlled trial (EudraCT 2010-023280-17) in the Paediatric Intensive Care Unit (PICU) of the Hospital Infantil Virgen del Rocío (Seville, Spain) between June 2011 and May 2013. We included patients aged 6 months to 14 years weighing 6 or more kg that had undergone elective surgery and in whom postoperative oral and/or enteral fasting with maintenance IV fluid therapy was prescribed for a minimum of 6h. We obtained written informed consent from the parents or legal guardians. The exclusion criteria were diabetes and refusal of consent. Patients that required changes in treatment, treatment for acute hypoglycaemia (glycaemia<60mg/dL) or whose consent for participation was withdrawn during the study were excluded from the final analysis. We used the Epidat 3 software to allocate patients randomly to one of two treatment groups, on a 1:1 ratio: the hypotonic (HT) group, which received 2/3 dextrose in 1/3 saline (51mEq of chloride, 51mEq of sodium and 33g of dextrose per litre of solution), and the isotonic (IT) group, which received 5% dextrose in 0.9% saline (154mEq of chloride, 154mEq of sodium and 50g of dextrose per litre of solution). We calculated fluid requirements by the Holliday-Segar method12 and did not add any other components to the administered solutions. Patient followup extended from the time of PICU admission to the development of oral/enteral tolerance and/or discontinuation of IV fluid therapy or discharge from PICU, for a maximum of 48h, with recording of plasma glucose levels at admission and at 8, 24 and 48h. We collected data on demographic characteristics and the duration and type of surgical intervention. During surgery, patients received isotonic fluids without dextrose.
The primary outcome was the plasma glucose level (in mg/dL). The secondary outcome was the incidence of hyperglycaemia. We defined hyperglycaemia as a plasma glucose level of more than 150mg/dL based on the findings of studies in adults and children that analysed the association of elevated glucose levels with morbidity and mortality in critical patients.10,13,14 Patients that had a plasma glucose level of less than 60mg/dL at any point in the study were treated with IV dextrose and excluded from the analysis. We did not use insulin to manage hyperglycaemia.
The plasma glucose levels were measured in the hospital's laboratory using a Cobas ISE analyser (Roche, Spain).
We conducted the trial in adherence to the principles of the Declaration of Helsinki and current law (Royal Decree 223/2004), following evaluation and approval by the Comité Autonómico de Ensayos Clínicos de Andalucía (the regional board that oversees clinical trials in Andalusia) and the Clinical Research Ethics Committee of our hospital.
We performed the statistical analysis with the software SPSS 20.0 for Windows (IBM). We have summarised quantitative variables using the mean and standard deviation, and qualitative variable using absolute frequencies and percentages. To assess associations between two variables, we built contingency tables and used the chi square test, the Yates chi square test or the Fisher exact test (for 2×2 tables with small cell sizes) as applicable. To assess whether the changes that occurred in certain variables at different time points in the followup differed between groups, we used the Student t test after verifying the random sampling, independence, normality and homogeneity of variance assumptions. If the assumption of homogeneity of variance was not met (Levene test), we used the Student t test with the Welch correction. If the assumption of normality was not met (Shapiro–Wilk test), we used the Mann–Whitney U test. When the normality assumption was not met, we calculated 95% confidence intervals for significant differences between means. We defined statistical significance as a p-value of less than 0.05.
ResultsThe final analysis included 130 patients: 60 in the HT group and 70 in the IT group. There were no differences in the baseline characteristics of patients in these groups, save in body weight (Table 1).
Characteristics of the sample under study at the time of admission.
| HT group (n=60) | IT group (n=70) | P | |
|---|---|---|---|
| Age (months) | 56±48.3 | 71.2±50 | .082 |
| Sex, n (%) | 0.721 | ||
| Male | 29 (48.3) | 37 (52.9) | |
| Female | 31 (51.7) | 33 (47.1) | |
| Peso (kg) | 18.2±11 | 23.7±15 | .034 |
| Duration of surgery (min) | 255±145 | 283±136 | .266 |
| Type of surgery, n (%) | |||
| Neurosurgery | 17 (28) | 16 (22.8) | |
| Trauma | 14 (23) | 19 (27) | |
| Tumour resection | 9 (15) | 14 (20) | |
| Maxillofacial/ENT | 9 (15) | 12 (17) | |
| Respiratory system | 7 (11.6) | 5 (7) | |
| Gastrointestinal | 3 (5) | 3 (4) | |
| Other | 1 (1.6) | 1 (1.4) | |
| Mean volume in operating theatre (mL) | 1146.58 | 1297.04 | .563 |
| Dextrose (mg/dL) | |||
| Pre-surgery | 92.6±26.8 | 90.4±23.3 | .916 |
| Post-surgery | 125±50.2 | 140.6±89.3 | .674 |
ENT, ear, nose throat; HT, hypotonic solution; IT, isotonic solution.
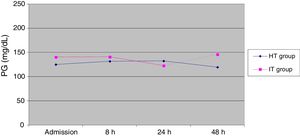
The mean glucose levels were higher in the IT group compared to the HT group throughout the study, except at 24h, where the mean was higher in the HT group, although the differences between groups were not statistically significant (Table 2, Figs. 1 and 2).
Mean plasma glucose levels in each group at each time point.
| HT group (n) | IT group (n) | P | |
|---|---|---|---|
| Admission | 125.1±50.2 (60) | 140.67±89.3 (70) | .67 |
| 8h | 131.76±50.8 (58) | 141.17±43.2 (66) | .45 |
| 24h | 132.52±85.7 (27) | 123.03±37.9 (29) | .37 |
| 48h | 119.71±78.2 (7) | 146.22±50.8 (9) | .08 |
HT, hypotonic solution; IT, isotonic solution; n, number of patients that met inclusion criteria.
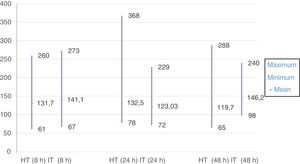
In the IT group, the mean plasma glucose level peaked at 8h from initiation of IV fluid therapy at 273mg/dL, while in the HT group, the mean peaked at 368mg/dL at 24h.
The lowest plasma glucose level in the study occurred in a patient in the HT group at 8h from admission, with a value of 61mg/dL. The minimum level in the IT group also occurred at this time point, with a value of 67mg/dL.
We found no significant differences between the IT and the HT groups in the frequency of hyperglycaemia at any point of the study.
At 8h, 26% of patients in the HT groups had plasma glucose levels of 150mg/dL or higher compared to 21.3% of patients in the IT group (P<.63).
At 24h, 20% of patients in the HT group and 22.7% of patients in the IT group had hyperglycaemia (P<.8).
At 48h, we observed plasma glucose levels of 150mg/dL or higher in 19% of patients in the HT group versus 23.1% in the IT group (P<.78).
DiscussionGlucose is the main source of energy for the cells in the human body, and therefore maintenance of optimal glucose levels is of utmost importance. Children are at higher risk of hypoglycaemia compared to adults due to their relatively greater glucose requirements and the particular characteristics of the intermediary metabolism in the early stages of life. The brain is the organ that suffers most from low glucose levels. Newborns and infants are particularly vulnerable groups in which hypoglycaemia carries a higher risk of neurologic sequelae. According to the guidelines of the American Diabetes Association (ADA),15 hyperglycaemia is defined as an 8-h fasting plasma glucose level of ≥126mg/dL.
Hyperglycaemia is one of the most frequent metabolic changes in hospitalised patients. Stress states such as inflammation, shock, hypoxia, major burns or polytrauma trigger adaptive metabolic and circulatory responses collectively known as systemic inflammatory response syndrome, with release of positive mediators of inflammation (proinflammatory cytokines) and neurohormones (insulin-antagonistic or counterregulatory hormones) with the purpose of ensuring an adequate supply of energy and oxygen to the tissues. Activation of these mechanisms produces an increase in neoglucogenesis in the liver and peripheral insulin resistance, leading to a state with elevation of plasma glucose known as stress hyperglycaemia. In this type of hyperglycaemia, ketosis is usually absent and the levels of glucose normalise once the acute illness that triggered the episode resolves.13
Hyperglycaemia is not free of complications. It can delay wound healing, increase the risk of infection, impair white blood cell function or cause osmotic diuresis that may result in secondary dehydration or electrolyte imbalances.
Like hypoglycaemia, hyperglycaemia can damage the brain. In the presence of ischaemia or hypoxia, cerebral blood flow decreases, causing lactic acidosis and changes in neural function that may even result in cell death. There is evidence in the paediatric literature that suggests that hyperglycaemia is associated with poorer neurologic outcomes in episodes of ischaemia or hypoxia and with increased morbidity and mortality in critically ill children.10,11 This underscores the importance of closely monitoring glucose levels and warrants the use of insulin therapy in certain cases to maintain stable glucose levels, preventing sharp rises.13,16 However, the target plasma glucose level in critical patients has not been clearly defined. Traditionally, the approach to the management of hyperglycaemia has been to treat it when the plasma glucose level exceeded the renal threshold of glucose reabsorption (180mg/dL), which would result in osmotic diuresis. This approach was justified by the greater priority that was given to preventing hypoglycaemia and its potential deleterious effects over maintaining stricter glycaemic control.10
However, several studies in adults have demonstrated that maintenance of lower glucose levels is associated with decreases in morbidity and mortality. The target ranges differed between studies: 108–144mg/dL (<150mg/dL),14 80–140mg/dL17 or, more recently, 80–110mg/dL.18
Although the paediatric literature is not as extensive as the literature on adults, studies performed in paediatric critical patients have found similar results, with a lower mortality and shorter lengths of stay in patients with plasma glucose levels of less than 150mg/dL.10,11 However, researchers also emphasise the risks of hypoglycaemia in patients under tight glycaemic control (with a blood glucose target of <126mg/dL) and the differences in the type of patients, age and underlying diseases,16 which have so far precluded the establishment of an optimal and universal cut-off point. In a study published in 2004, Srinivasan et al.10 reviewed a sample of 1350 children that were admitted to the ICU and required mechanical ventilation or vasoactive infusion. They defined normoglycaemia as a plasma glucose level of less than 110mg/dL and hyperglycaemia as a level of more than 126mg/dL, and found that hyperglycaemia with plasma glucose levels of more than 150mg/dL in the first 48h of PICU stay was associated with a 3-fold increase in the risk of mortality, and that sustained hyperglycaemia with levels of more than 126mg/dL was associated with a 6-fold increase in the risk of mortality. In 2005, Faustino and Apkon19 assessed the prevalence of hyperglycaemia in 942 nondiabetic children admitted to their PICU and established 3 cutoffs for the definition of hyperglycaemia (of 120, 150 and 200mg/dL), finding hyperglycaemia in 75% of the patients applying the first cutoff, in 50.1% with the second cutoff and in 26.3% with the third cutoff, as well as increased morbidity in patients that had blood glucose levels of more than 150mg/dL in the first 24h from admission. In 2006, Wintergerst et al.11 made a retrospective review of the plasma glucose levels measured in a sample of children admitted to one PICU over a period of 13 months to assess the association between hypoglycaemia (defined as glucose level<65mg/dL), hyperglycaemia (whose prevalence was defined for 3 cut-off points: 110, 150 and 200mg/dL) and the fluctuations in glucose levels in each patient during the followup. The prevalence of hyperglycaemia was 86.7% applying the first cutoff, 61% with the second cutoff and 35% with the third cutoff. They found that hyperglycaemia was positively correlated to both mortality and PICU length of stay, with a mortality rate of 9.9% in patients with glucose levels of more than 200mg/dL, of 7.4% in patients with maximum levels above 150mg/dL, of 5.7% in patients with maximum levels above 110mg/dL, and of only 1% when maximum levels were below 110mg/dL. They found hypoglycaemia in 18.5% of patients, and hypoglycaemia was associated with increased morbidity and mortality.
In our study, we found that the mean plasma glucose levels exceeded the threshold used by the ADA to define hyperglycaemia (≥126mg/dL) at every time point, with no differences between the two groups. However, when we defined hyperglycaemia as a level of 150mg/dL or greater, we found a lower overall prevalence of hyperglycaemia, with the prevalence amounting to 23.7% of the total sample at 8h, 21.3% at 24h and 21.1% at 48h, with no significant differences between the HT and the IT groups at any point.
The mean plasma glucose levels were higher in the group that received higher concentrations of glucose, except at the 24-h time point. Furthermore, we observed the highest individual glycaemia values in the HT group at 24 and 48h in patients that had received lower amounts of dextrose intravenously. When we analysed factors other than the administered amounts of IV dextrose to try to explain these results, we concluded that surgical stress did not seem to be involved, as there were no differences in the mean duration of surgery and the distribution of patients by type of surgery was similar in both groups. When we analysed these two cases individually, we found that these patients had plasma glucose values in the normal range before surgery (113 and 77mg/dL), and levels that were within the expected range for stress hypoglycaemia at admission and at 8h post-surgery (139 and 84mg/dL), although the duration of surgery was above the mean in one of these patients (900min). We need to take into account that our analysis did not consider another factor, the administration of drugs that could raise glucose levels, which may have explained these results.
We ought to highlight the increase in glucose levels observed between the preoperative period and the postoperative period, the latter recorded at the time of admission to the PICU in both groups. The mean glucose level rose from 92.6±26.8mg/dL before surgery to 125.1±50mg/dL after surgery in the HT group, and from 90.5±23.2mg/dL before surgery to 140.6±89.3 after surgery in the IT group. This could be explained by the rise in plasma glucose associated with the hypermetabolic stress response produced by surgery, as there is widespread agreement not to include dextrose in the IV fluids administered during surgery except in patients at high risk of hypoglycaemia.9
Analysing the association between hyperglycaemia and morbidity and mortality was not one of our objectives when we designed this study; our objective was rather to assess whether there were any differences in glucose levels between the two groups that received IV solutions with different concentrations of dextrose (5% vs 3.3%). Our findings reflect a similar prevalence of hyperglycaemia in both groups in the first 48h post surgery despite the different concentrations of dextrose administered to each group. However, as we have already mentioned, postoperative glucose levels may be affected by factors such as the hypermetabolic stress response or the use of steroid drugs (indicated for prevention of post-extubation stridor or in neurosurgical patients at risk of raised intracranial pressure), which we did not document or analyse in this case series.
Thus, we may conclude that administration of fluids with a 3.3% dextrose concentration achieves plasma glucose levels similar to those obtained in patients that receive saline with 5% glucose, with a similar incidence of hyperglycaemia, so that use of the lower concentration could be equally efficacious in maintaining glucose levels within the target range. It is also important to take into account the additional raises in glucose levels that may result from other factors and contribute to stress hyperglycaemia.
One of the main limitations of this study concerned its design, as patients could be discharged or start oral or enteral feeding before the end of the maximum follow-up period. This led to significant losses during the followup and a small sample size at the 48-h time point, which limits the statistical power of some of the results. Another limitation is that we did not collect data on other factors that may affect blood glucose levels, such as steroid therapy, as we noted above. We are currently designing another study in our hospital that will address this subject more specifically and resolve these limitations.
FundingThis study was part of a clinical trial that was funded by a public non-commercial research grant awarded by the Spanish Ministry of Health and Social Policy in 2010 (grant number EC10/184) for a total of 60000 €.
Conflicts of interestThe authors have no conflicts of interest to declare.
We want to thank all the staff members and colleagues of the Paediatric Intensive Care Unit of the Hospital Infantil Virgen del Rocío in Seville for their collaboration in the execution of the study and data collection. We also thank Fernando Pérez Martínez, professor of clinical research, Clara María Rosso Fernández, specialist in clinical pharmacology and head of the Clinical Research and Clinical Trials Unit of the Hospital Virgen del Rocío of Seville, and Carlos Rial Garrido, Project Manager of the Fundación Pública Andaluza para la Gestión de la Investigación en Salud, for their support and guidance in the design and implementation of the study.
Please cite this article as: Martínez Carapeto I, López Castilla JD, Fresneda Gutiérrez R. Comparación de niveles de glucemia postoperatoria usando sueros con diferente concentración de glucosa. An Pediatr (Barc). 2018;89:98–103.
Previous presentation: This study was presented as an oral communication and received the First Prize for the best oral communication at the XXXII Congress of the Sociedad Española de Cuidados Intensivos Pediátricos; May 4–6, 2017; Granada, Spain.