In few previous studies, it has been reported that hypercalciuria is associated with some types of congenital anomalies of the kidney and urinary tract (CAKUT), namely ureteropelvic junction obstruction, vesicoureteral reflux or simple renal cysts. In addition, one higher prevalence of hypercalciuria and/or urolithiasis has been described in their family members compared to the general population. This study was carried out to find out whether children with unilateral renal agenesis (URA) have these features previously described in other CAKUT types.
MethodsIn a descriptive and multicenter study we studied the prevalence of hypercalciuria, hypocitraturia and urolithiasis in 67 children (43 males and 24 females) with URA and their families.
ResultsThe two metabolic anomalies that promote stone formation were observed in 26 children (38.8%), distributed as follows: hypercalciuria in 16, hypocitraturia in 9, and both hypercalciuria and hypocitraturia in 1. Eight children (11.9%) suffered renal colic during follow-up. Familial history of urolithiasis was found in 42/67 children (62.7%): in 12 of the first-degree relatives, in 15 of the second degree relatives and in 15 patients both in the first-degree as in their second degree relatives. In contrast, in historic control group, only in 28.1% of families at least one member had urolithiasis.
ConclusionOur results show that the prevalence of hypercalciuria and/or hypocitraturia is greater in pediatric patients with URA than in the general population. Likewise, the prevalence of urolithiasis in the families of these children is also higher than that in the general population.
En escasos trabajos previos, se ha comunicado que puede observarse la presencia de hipercalciuria en pacientes portadores de algunos tipos de CAKUT como estenosis pieloureteral, reflujo vesicoureteral o quistes renales simples. Además, se ha descrito una prevalencia mayor de hipercalciuria y/o urolitiasis en los miembros de las familias de esos niños con algunos tipos de CAKUT, en comparación con la población en general. El presente estudio se llevó a cabo para averiguar si los niños con agenesia renal unilateral (ARU) tienen las características descritas anteriormente en otros tipos de CAKUT.
MétodosEn un estudio descriptivo y multicéntrico se determinó la prevalencia de hipercalciuria, hipocitraturia y urolitiasis en 67 niños (43 hombres y 24 mujeres) con ARU y sus familias.
ResultadosEn 26 niños (38,8%) se observaron las dos anomalías metabólicas que favorecen la formación de cálculos renales distribuidos de la siguiente manera: hipercalciuria en 16, hipocitraturia en 9 y tanto hipercalciuria como hipocitraturia en 1. Ocho niños (11,9%) padecieron un cólico renal durante el tiempo total de seguimiento. Una historia familiar de litiasis urinaria se encontró en 42/67 de los niños (62,7%): en familiares de primer grado en 12 de ellos, en familiares de segundo grado en 15 y en ambos grados de familiares en los otros 15. En contraste, en el grupo de control histórico, solamente en 28,1% de las familias, al menos, un miembro había tenido urolitiasis.
ConclusiónNuestros resultados muestran que la prevalencia de la hipercalciuria y/o hipocitraturia en pacientes pediátricos con ARU es mayor que en la población general. Asimismo, la prevalencia de urolitiasis en las familias de estos niños es también mayor que en la población general.
Association between urolithiasis and congenital anomalies of the kidney and urinary tract (CAKUT) has been described in children and adults since the 1920s.1 The cause of susceptibility to lithiasis was believed to be either: urinary stasis,2 reduced urinary flow and urinary tract infection in which Proteus was the most common microorganism.3 Urinary stasis is generally assumed to play a major part in the pathogenesis of nephrolithiasis associated with distorted renal anatomy due to a delayed washout of crystals and risk of urinary infections. The reported frequency of genitourinary anomalies in children with urolithiasis is between 19.1%4 and 29.8%.5 However, the exact pathogenic relationship between urolithiasis and CAKUT remains unclear.
We reported a higher incidence of hypercalciuria in children with vesicoureteral reflux (VUR),6 ureteropelvic junction obstruction7 and simple renal cysts8 in the general population, and a higher positive family history of urolithiasis.6–8 This implies that children of parents with urolithiasis, and/or with a family history of urolithiasis, would have increased susceptibility to have a kidney development abnormality. These children also inherit hypercalciuria that could also favor formation of kidney stones.
The present study was conducted to find out if children with unilateral renal agenesis (URA) have a higher prevalence of hypercalciuria, hypocitraturia and/or a familiar history of urolithiasis.
Subjects and methodsPatientsThis descriptive and multicenter study included 67 children (43 males, 24 females) with URA who were reviewed at Spanish Pediatric Nephrology Units of “Hospital Nuestra Señora de Candelaria” (Tenerife) (n=35), “Hospital Universitari Sant Joan de Reus” (Tarragona) (n=16), “Hospital de Fuenlabrada” (Madrid) (n=10) and “Hospital General Universitario Santa Lucía” (Cartagena) (n=8). The age at the time of the study was 10.0±5.84 years (range: 0.16–21.5). The patients were controlled in each hospital from the postnatal ultrasound confirmation of the diagnosis of URA that had performed in utero, i.e. since the first weeks of life.
URA was located on the left side in 41 cases, and on the right side in 26. The diagnosis of URA was made by means of renal ultrasound and DMSA scan. URA was associated with vesicoureteral reflux in three patients. No other urinary tract malformations were found. None of them had nephrocalcinosis or renal dysplasia morphological signs. During follow-up, 27 children suffered from urinary tract infections. All patients were free of infection at the time of simple urine collection. None of the patients were diagnosed with chronic renal failure. None of the children showed elevated levels of plasma creatinine, hypertension, or signs of distal renal tubular acidosis. We asked parents and/or other relatives about family history of urolithiasis of the 67 children. There was no background of consanguinity in the family histories.
MethodsThe urine collected corresponded to non-fasting samples in which calcium, citrate and creatinine were determined. In accordance with criteria set by the Spanish Paediatric Nephrology Association, hypercalciuria is diagnosed in children between 1 and 2 years of age when the calcium/creatinine ratio (UCa/UCr) from two individual, consecutive measures is greater than 0.47mg/g.9 Based on the results of So et al., hypercalciuria in children between 2 and 4 years of age was diagnosed when UCa/UCr was greater than 0.28mg/mg.10 In children older than 4 years of age, the diagnosis of hypercalciuria was made when the UCa/UCr was greater than 0.20mg/mg,11 a value that corresponds to the 95th percentile of a sample made up of 100 healthy children, from a previous study conducted by one of our groups.12 Based on Stapleton and Kroovand's criteria, the hypocitraturia diagnosis criterion is a citrate/creatinine ratio less than 400mg/g.13 In patients older than 14 years, the hypocitraturia diagnosis criterion is a citrate/creatinine ratio less than 250mg/g.14
The UCa/UCr ratio in non-fasting single spot urine was measured, in addition, to the parents of 29/68 of the patients without changing their diets. In these adults, hypercalciuria was diagnosed when UCa/UCr was higher than 0.2mg/mg, according to the average value previously found in our adult population from previous study conducted by one of our groups (UCa/UCr=0.12±0.04mg/mg).6
The results of urinary concentration test and urinary elimination of albumin were collected from the medical records. The normal values used as reference to the maximum urine osmolality15,16 and the albumin/creatinine ratio17 have been previously published.
The frequency of family history of urolithiasis in parents and other relatives of our URA patients was compared with a historical control group conducted in one of the hospitals participating in the study.18
Analytical procedures and renal function testsIn the laboratories of each of the hospitals participating in the study, urinary creatinine was determined by the creatininase method, calcium was measured by photometric assay, urinary citrate was assessed by the citrate lyase method and urinary albumin was measured by a nephelometric technique (Array).
Renal concentrating capacity was determined after administration of 20μg of desmopressin intranasal or 0.12mg (120μg) of oral desmopressin lyophilisate (MELT) that dissolves immediately in the mouth. After emptying the bladder three urine samples were collected at 90min intervals. The highest osmolality value obtained was taken as the test result.15,16 Urinary osmolality was determined by measuring the freezing point depression in an Osmostat Osmometer (Menarini).
Statistical methodsKolmogorov–Smirnov test was used to study the distribution of the variables. When they fit a normal distribution, a notation of average value and standard deviation is used. The other quantitative variables were expressed in terms of median and interquartile ranges. Basic statistics were performed using SPSS statistical software (SPSS V 19.0, SPSS Inc., USA).
All of the procedures and protocols followed during this study meet the ethical, administrative, and data protection requirements imposed by the Paediatrics Departments of the hospitals participants, which are established in accordance with the law of Spain. All parents gave informed consent.
ResultsMetabolic disorder was observed in 26 children: hypercalciuria in 16 children (11M, 5F), hypocitraturia in 9 cases (5M, 4F) and both hypercalciuria and hypocitraturia in one patient. Thereby we observed any risk of lithiasis in 38.8% of children with URA. Eight children (11.9%) suffered renal colic during follow-up (four with hypercalciuria and four with hypocitraturia).
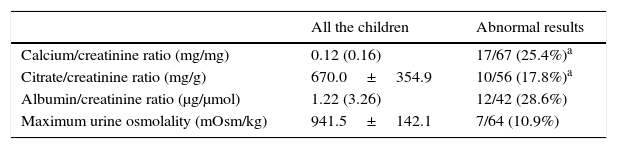
Table 1 shows the values of the studied parameters as well as the percentage of children with metabolic or functional anomalies.
Metabolic and functional parameters. Abnormal results.
The familial history of urolithiasis was found in 42/67 children (62.7%): 12 in the first-degree, 15 in the second degree and in 15 patients both in the first and second degree relatives.
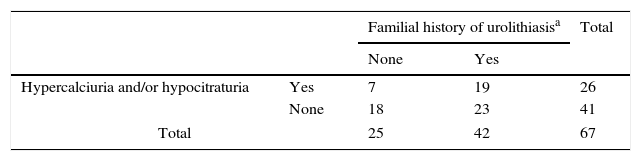
Familial history of urolithiasis and the concomitant existence of hypercalciuria and/or hypocitraturia in patients with URA were observed in 19 families. In another 23 families only history of urolithiasis existed in children's relatives. In another 7 families the patients were carriers of metabolic anomalies potentially causing kidney stones. In 23 additional families, only history of urolithiasis existed in children's relatives. In 7 additional families the patients were carriers of metabolic anomalies potentially causing kidney stones. In summary, some sort of manifestation of hypercalciuria or urolithiasis occurred in 49 families (73.1%) of URA patients (Table 2).
Relationship between familial history of urolithiasis, hypercalciuria and/or hypocitraturia in the patients with URA.
| Familial history of urolithiasisa | Total | |||
|---|---|---|---|---|
| None | Yes | |||
| Hypercalciuria and/or hypocitraturia | Yes | 7 | 19 | 26 |
| None | 18 | 23 | 41 | |
| Total | 25 | 42 | 67 | |
We have previously reported a greater prevalence of hypercalciuria in pediatric patients with vesicoureteral reflux (VUR) than in the general population, and hypothesized that urolithiasis in patients with VUR should have a metabolic origin. We also reported that hypercalciuria was inherited as an autosomal dominant trait, although with a higher probability of inheritance from the mother.6
Recently, Madani et al. confirmed that the frequency of hypercalciuria was higher in pediatric patients with VUR than in healthy children, although relatives were not studied.19
The incidence of urolithiasis in patients with ureteropelvic junction obstruction (UPJO) is 16–44.7%20 with a 70-fold increased risk for developing kidney stones.21 In a previous survey, Husmann et al. reported that 76% of patients with UPJO and simultaneous non-struvite renal calculi presented an identifiable metabolic abnormality.22 Hypercalciuria has been the most frequently reported metabolic disturbance in patients with UPJO21,22 although others, such as hypocitraturia and hyperoxaluria, have also been found.20 No mention is made of the origin of hypercalciuria and the other metabolic disturbances causing calculi present in patients with UPJO.
We conducted a study to find out if children with UPJO have higher prevalence of hypercalciuria and whether their family members were affected by hypercalciuria and/or urolithiasis.7 Hypercalciuria was found in 17/27 children (63%), 15 of them (88%) had a familial history of urolithiasis. Concerning the 10 children without hypercalciuria, seven of them (70%) had a familial history of urolithiasis. Prevalence of both urolithiasis and hypercalciuria, was not influenced by gender. In summary, in concordance with previous data,21,22 our results show that the prevalence of hypercalciuria is greater in pediatric patients with UPJO than in the general population and seems to be of genetic origin.7
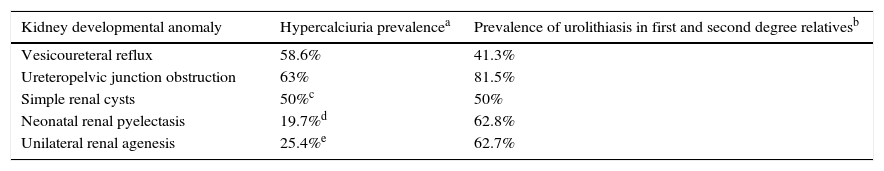
Subsequently, we obtained results similar to those observed in the cases of VUR and UPJO in children diagnosed of simple renal cysts11 and, in neonatal renal pyelectasis.18 Therefore, these asymptomatic morphological anomalies (renal cysts and, neonatal renal pyelectasis), while lacking clinical significance; behave as other malformations potentially more harmful in terms of its association with genetic urolithiasis (Table 3).
Frequency of hypercalciuria in patients and urolithiasis in their relatives in the present report and in our reports carried out in children with four types of kidney development anomalies (Refs. 8).
| Kidney developmental anomaly | Hypercalciuria prevalencea | Prevalence of urolithiasis in first and second degree relativesb |
|---|---|---|
| Vesicoureteral reflux | 58.6% | 41.3% |
| Ureteropelvic junction obstruction | 63% | 81.5% |
| Simple renal cysts | 50%c | 50% |
| Neonatal renal pyelectasis | 19.7%d | 62.8% |
| Unilateral renal agenesis | 25.4%e | 62.7% |
Unilateral renal agenesis accounts for 5% of CAKUT, and it is defined as congenital absence of renal parenchymal tissue and arises from an error in organogenesis. Multiple factors are thought to be implicated in the pathogenesis of renal agenesis including mutations in various genes important in renal development.23 The majority of patients are asymptomatic, so it is usually diagnosed incidentally as a result of an antenatal ultrasound or as part of an evaluation for a urinary tract infection. Unilateral renal agenesis can be accompanied by other CAKUT (32%) and no renal anomalies. Vesicoureteral reflux is the most common finding (24% of patients).24
As in other CAKUT anomalies, in URA patients the formation of kidney stones has been observed.1,2,25 To our knowledge this is the first study that has studied metabolic disturbances causing calculi in children diagnosed with URA.
In our patients, hypercalciuria frequency was 25.4%. We previously reported the prevalence of hypercalciuria in control children in Tenerife island (3.8%).12 In other countries the hypercalciuria prevalence is reported between 0.6%26 and 12.5%.27 Adding the patients with hypocitraturia, the frequency of metabolic abnormalities causing of urolithiasis in URA patients rises to 38.8%. We do not know of any hypocitraturia prevalence studies that have been performed in a control pediatric population.
The frequency of urolithiasis in first and second degree relatives of our patients (62.7%) was also higher than that which we have described in our community (28.1%).18 The authors are aware that the use of a historical series of prevalence of urolithiasis, although recent, is a limitation of our study. The prevalence of urolithiasis in the adult population is well studied in the medical literature, but we not know of any studies where first and second degree relatives are studied simultaneously.
It is also difficult to explain the reason why some patients were carriers of hypocitraturia and not of hypercalciuria. In our experience we have seen that in many children with idiopathic hypercalciuria urinary calcium excretion can normalize around adolescence and, at the same time, reduce the citraturia. Also, we have observed, in the families of some children with idiopathic hypercalciuria, parents with urolithiasis had hypocitraturia and, on the other hand, a normal urinary calcium excretion. It seems as if they were two sides of the same process. It has been described that idiopathic hypercalciuria may be associated with hypocitraturia in absence of dRTA.28 Longitudinal studies aimed at resolving this issue are required.
The renal malformation in which metabolic abnormalities has been most demonstrated to causes kidney stones is medullary sponge kidney (Lenarduzzi–Cacchi–Ricci disease). In this disorder, the most common abnormality is idiopathic hypercalciuria (88%).29 In another study 65 of 97 patients (67%) had at least one stone risk factor as hypercalciuria, hypocitraturia, hyperuricosuria or hyperoxaluria.30 Recently, Fabris et al. studied the families of 50 patients with medullary sponge kidney. Twenty-seven probands (54%) had 59 first- and second-degree relatives of both genders with medullary sponge kidney or urolithiasis.31 The authors concluded that their study provides strong evidence that familial clustering of medullary sponge kidney is common, and has an autosomal dominant inheritance, a reduced penetrance and variable expressivity. The question is whether those family members have a milder form of medullary sponge kidney in some cases or simply kidney stones in the other, something similar to what we have described in relatives of children with other CAKUT malformations.6,7
The type of inheritance described by Fabris et al. in the article previously mentioned31 is similar to that which we have communicated in our patients with VUR6 and UPJO.7 In summary, and according to our hypothesis, medullary sponge kidney would not be a renal disease sensu stricto, but another renal malformation characterized by dilation of the collecting tubules which would share with other CAKUT anomalies (RVU, UPJO, simple renal cysts, URA) a higher prevalence of hypercalciuria and/or urolithiasis in the family members which is genetically transmitted to their descendants, carriers of one or several among many possibilities of CAKUT development anomalies. The hypothesis that medullary sponge kidney is a CAKUT malformation was suggested in two previous reports.32,33
Finally, it is important to remember that idiopathic hypercalciuria has been described, also in another kidney malformation: the horseshoe kidney.34 It is also intriguing that in patients with an abnormality of glomerular development, the thin basement membrane nephropathy, has also been described with hypercalciuria, hyperuricosuria and nephrolithiasis.35 Even thin basement membrane nephropathy can be associated with simple renal cysts.36
For many years in medical literature it could be read that the pathophysiologic mechanism of stone formation in children and adults with congenital urinary tract abnormalities were the urine stasis and infection. The mechanisms are necessarily more complex. We believe that the anomalies of the kidney development and genetic predisposition to produce kidney stones have a genetic connection that must be discovered in the next few years. In genetic hypercalciuric stone-forming rats an increase has been reported in the number of receptors for vitamin D (VDR) both in the intestine and bone. Favus et al. showed in humans that peripheral monocytes of patients with idiopathic hypercalciuria have an increase in the number of vitamin D receptors,37 i.e. the same as the hypercalciuric rats. Therefore, an increase in the functional capacity of the complex calcitriol-VDR would be the most likely cause of idiopathic hypercalciuria.38
Somehow vitamin D is involved in the kidney intrauterine development? In 2001, Wagner et al. showed that the WT1 gene product transcriptionally activates VDR expression in human embryonic kidney cells.39WT1 gene expression is critical for genitourinary development. Homozygous disruption of this gen in mice caused agenesis of the kidneys, likely as a result of a loss of metanephric blastemal cells.40 In humans, mutations in WT1 gene, as well as Denys-Drash syndrome, originates renal agenesis. In their paper, Wagner et al. sounded a role for the vitamin D endocrine system in the regulation of renal cell growth and differentiation during development since up regulation of VDR by the WT1 transcription factor may mediate apoptosis of renal embryonic cells in response to 1,25(OH)2D3.39 Is this Ariadne's thread that will help us to solve the labyrinth of the association between kidney stones and CAKUT anomalies?
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Garcia Nieto V, Huertes Díaz B, Escribano Subias J, Alarcó Alacio MT, Gonzalez Rodríguez JD, Cabrera Sevilla JE, et al. Agenesia renal unilateral. Nuevos argumentos acerca de la relación genética entre la urolitiasis y las malformaciones renales. An Pediatr (Barc). 2016;85:240–246.