EuroNeoStat is a European project, financed entirely by one million Euros from the European Commission Health and Consumer Protection Directorate General (DG Sanco), within the VI Research Framework Programme for 2006 to 2008. Neonatal units from 23 European countries work together on this project, which is led from our country. Its overall objective is to create a European information system that monitors the short- and long-term outcomes of extremely low birth-weight infants (ELBWI) attended at the units taking part, in order to improve the quality and safety of care provided and so improve their development.
Currently, premature birth must be a health priority, given its high prevalence and its grave family, social, individual and health-care consequences, as well as its economic impact. These babies not only require long stays in neonatal intensive care units (NICUs), but often require rehospitalisation, along with multiple preventive, therapeutic and rehabilitatory interventions, which drain the always limited health-care resources available. All this means that this project is extremely relevant both for health-care delivery and in social and economic terms.
Although the end aim of Perinatology is to avoid premature birth, this target has not been attainable. In fact, the same as in Europe as a whole 1, premature birth rates have increased in our country, such that in three decades they have almost doubled (from 4-5 % to the current 7-8 %) 2. In addition, ELBWI, under 1,500 grams at birth, though only accounting for 1-2 % of all births, have high specific mortality (15-20 %) and morbidity (10-20 %), which means they contribute greatly both to neonatal and child mortality 3 and to childhood disability. It should also not be forgotten that, although these rates depend on maternal health and the perinatal care received, specific morbi-mortality is the outcome, among other factors, of the quality of neonatal care.
In NICUs, monitoring and life-support techniques have not only increased survival 4, but have avoided some of these patients suffering injuries that would lead eventually to long-term disability. Despite this, an overall 10 % of ELBWI are gravely disabled; and a further 10 %, moderately disabled 5,6. In addition, 20-30 % of the most immature babies have a cognitive or attention deficiency or delayed psycho-motor development 7.
If the outcomes are to be improved, then those factors of risk that can be modified must be identified, the care process needs to be analysed and the results for one unit, region or country need to be compared with reference populations. In addition to intra-institution analysis, as long as explicit and homogeneous indicators and criteria are followed, inter-institution comparisons can also be conducted 8.
Networking
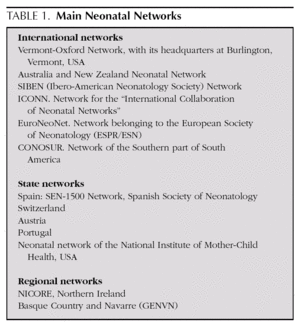
The systematic prospective gathering of homogeneous indicators enables inter-centre comparisons to be made, for use in epidemiological control and evaluation of the quality of care delivered to these patients at such high risk. Various experiences of networking exist, which have tackled and resolved the problem of specific information on ELBWI (table 1). The best known of these is the Vermont-Oxford Neonatal Network (VON) in the United States 8. Among us, most Spanish NICUs take part in the SEN-1500 initiative coordinated by the Spanish Neonatology Society (www.se-neonatal.es) and some take part in the EuroNeoNet (www.euroneonet.org).
All these neonatal networks are voluntary collaborations between neonatal units, with a data-gathering structure based on close monitoring of each case till death or hospital discharge. They all collect data on risk factors, interventions and complications in the care process, and on outcomes, all of which are variables closely linked to the quality of health-care delivery. The value of all this is based on the following premises:
1)The mortality of ELBWI reaches 50-60 % of neonatal mortality and 40-50 % of infant mortality, figures which rise still further for long-term morbidity.
2)ELBWI only account for 1-2 % of all neonates, and are wholly identifiable, as they are all born in hospital and attended in NICUs.
3)Their outcomes are related to initial blood flow (degree of immaturity), as well as to the quality of perinatal and neonatal care received.
4)There are highly effective antenatal and perinatal strategies (e.g. corticoids and exogenous surfactant) that are not always used 9. That is to say, there are differences in strategies for managing these patients and room for improvement in currently used procedures.
5)Survivors may present with neurological or respiratory disabilities that require prolonged follow-up with multiple therapy and rehabilitation interventions and/or hospital readmission.
EuroNeoNet
The EuroNeoNet (European Neonatal Network) was created in 2002 as an information system on ELBWI and is linked to the European Society of Neonatology/European Society of Paediatric Research (ESN/ESPR; www. espr.info). Its mission is to try to see that every ELBWI cared for in Europe receives the best neonatal care possible, regardless of his/her place of birth. To achieve this, EuroNeoNet is based on four initiatives, the pillars of the virtual platform:
1)Standardised comparison of perinatal outcomes and of monitoring of each participating unit with the rest (external auditing or "benchmarking"), without data identifying individuals or institutions being included in any case.
2)Promotion of the safety of patients attended.
3)Ongoing virtual training of professionals ("E-learning").
4)Independent clinical trials, not promoted by the pharmaceutical industry.
To overcome the operational difficulties of implementing information systems, new Internet information and communication technology is essential. It enables us to:
1)Introduce and send data directly by e-mail or straight to the web site, with guaranteed protection meeting all possible requisites for data confidentiality and security.
2)Extract autonomously and by computer data for quality control.
3)Spread more quickly and efficiently initiatives to improve health-care quality.
With all the above, the aim is to create a culture of continual improvement of health-care quality, by means of the networking of the professionals involved, which promotes the safety of the patients attended in the NICUs, prioritises health care focusing on the family and on caring for the neonate's development, and favours the spread of prophylactic and therapeutic strategies based on the best scientific evidence available.
EuroNeoStat
The management committee of EuroNeoNet, with the experience accumulated since 2002, decided to put forward a proposal for developing an epidemiological project to the 2005 Public Health Call of the EC (proposal number A/790698, contract 2005/16), under the title "European Information System for monitoring short- and long-term morbidity in order to improve quality of care and patient safety for extremely low birth-weight infants". The initiative was approved for the period 2006-8 and is known by the acronym, EuroNeoStat.
This European project is, as well as an epidemiological watch study of the EC's Health and Consumer Protection Directorate General on the consequences of premature birth, a project to research into health systems on the health care delivered to ELBWI in the units taking part. The strategic objective is to develop an Information System to control and improve the quality of health care delivered to very immature neonates (< 32 weeks gestation) or extremely low birth-weight infants (< 1,500 gr.). To achieve this, a number of neonatal and post-neonatal indicators of morbi-mortality were created, with the aim of:
1)Gathering data to compare outcomes from the neonatal units taking part with those from other institutions (external auditing). This is designed to identify areas with opportunities for improvement of results of the care process, as well as to have data available to make clear the success of the initiatives undertaken (internal auditing).
2)Developing indicators so that health organisations can evaluate health programmes and priorities for planning, promotion and evaluation of short- and long-term care of ELBWI.
3)Finding the clinical variability of the care process and its outcomes, so as to analyse the best way of applying and promoting health care.
4)Pushing forward consensus in the health policies and strategies to be used in caring for ELBWI.
Concretely, the aim is to obtain data on at least 4,000 ELBWI per year, from at least 50 NICUs in 23 European countries, coming both from the institutions that are part of the consortium (see Appendix) and neonatal networks, whether local, regional (Liverpool, Basque Country and Navarre etc.) or national ones (Austria, Switzerland, Portugal etc.) that are prepared to contribute to the project.
As well as perinatal data, and following the line marked out by Dr. Johnson 10, a minimum of monitoring data will be developed and introduced in selected units to evaluate the state of health of infants who survive for 24 months, at corrected postnatal age.
Three work-groups have been set up to take the project forward: on perinatal data, on monitoring and on safety of the patient, led by Adolf Valls i Soler, Michael Weindling and Harry Molendijk, respectively. In addition, the Bilbao coordinating centre has taken on a graduate in Statistics, a Computer expert, a clerical assistant, a secretary and an epidemiologist who works half-time.
EuroNeoSafe
EuroNeoStat also includes an initiative, called EuroNeoSafe, to promote the safety of the ELBWI attended in the NICUs. This initiative aims to develop a culture that places the safety of these tiny patients first by minimising medication errors 11 or other mistakes, a frequent cause of neonatal morbi-mortality 12. As well as spreading information on these questions, it hopes to create a system of voluntary communication of adverse incidents or near-incidents. The purpose is not to find the guilty party, as error is human, but to analyse and clarify an incident's causes and put forward corrective mechanisms to reduce the frequency and consequences of this kind of error.
In summary, in order to tackle successfully initiatives aimed at improving outcomes in the process of perinatal care of the most immature and vulnerable neonates, collaborative networking, an attitude of constructive criticism and thorough comparative analysis of the outcomes and incidents in the health-care process are indispensable.
Correspondence: Dr. Adolf Valls i Soler.
Neonatal Unit. Hospital de Cruces.
Plaza de Cruces, s/n.
48903 Barakaldo-Bilbao, Bizkaia, Spain.
E-mail: avalls@hcru.osakidetza.net
Date sent: 15 May, 2006.