Introduction
Acute Rheumatic Fever (ARF), a non-suppurative sequelae of group A β -hemolytic streptococcal infection (GAS) has become a rare pathology in developed countries. However, every year, pediatric services from large urban centres continue to diagnose patients with ARF1,2. Moreover, it is difficult to establish the diagnosis, as many young physicians have never seen a patient with ARF during their training1,3-5. Transcontinental migration and changing virulence of certain strains of GAS may influence the epidemiology of ARF.
Regular reassessment of the epidemiology is essential in order to recognize and to diagnose promptly this disease5-7. Recent published literature contains only few studies with large number of pediatric patients. There is the feeling that ARF is more of an issue of the past, however we have found a consistent number of new cases each and every year over the past 15 years. This paper is meant to highlight the epidemiology and clinical characteristics of the cohort of children diagnosed with ARF in a modern North American/Canadian city.
Material and methods
Study population and data collection
A retrospective chart review of all children diagnosed between 1979 and 2005 with ARF in the pediatric tertiary care referral centres. Montreal (Canada) has two Pediatric hospitals: Sainte-Justine Hospital (a 452 bed pediatric hospital with 25,000 admissions and 300,000 total visits per year, 80,000 emergency room visits) and The Montreal Children's Hospital (MCH) a 214 bed pediatric hospital with 7,000 admissions and 120,000 total visits per year, 65,000 emergency room visits). Hospitalized children younger than 18 years of age diagnosed with ARF from January 1rst 1979 through December 31rst 2005 were included. Patient's charts were identified through medical records according to the codes 390-398 of the 9th Revision of the International Classification of the Diagnosis. Demographic characteristics, clinical presentation and outcome data were collected using a standardized data collection form by one investigator (AC). The following data was retained: Fever, if present in association with sore throat or on hospital admission; Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), PR interval on electrocardiogram, throat swabs results, antistreptolysin-O (ASO) and anti-DNAse B titres were recorded. Echocardiography results, occurrence of cardiac surgery and clinical evolution were also noted. When available, we reviewed pathological results and diagnostic confirmation done by direct observation of cardiac injuries.
Inclusion criteria
Any patient that fulfilled the Jones Criteria7 for ARF were retained. Carditis was considered with any new pathologic cardiac murmur, cardiomegaly, pericarditis or congestive heart failure or typical valve involvement at echocardiography8. Evidence of recent streptococcal infection was defined by Antistreptolysin-O antibodies (AS0) titres of 400 Todd Units or greater and Anti-deoxyribonuclease B (DNAse B) titres of 1/300 or greater2.
Exclusion criteria
Chorea explained by other causes, uncompleted Jones Criteria and patients with relapses episodes from remote rheumatic fever. Statistics on population in Montréal were obtained from Agence de la Santé et des Services Sociaux de Montréal9.
Statistical analysis
Data from the two hospitals were compiled and combined in the analysis using standard spreadsheet software (Microsoft Excel 2000tm and SPSS 11.0 for Windows). Descriptive statistics were calculated on the entire cohort except when mentioned otherwise. Logistic regression was done to establish whether certain covariates were associated with the different manifestations of ARF. Maximum likelihood estimates of regression coefficients were used to estimate odds ratios. Ninety-five percent confidence intervals were calculated for all estimates reported.
Results
Demographic characteristics
During the 27 years study period, 134 charts were selected, of which 36 were excluded because of a past history of ARF. The remaining 98 patients fulfilled the Jones criteria of ARF7 and were included in the analysis: 47 children from Sainte-Justine Hospital, and 51 from The Montreal Children's Hospital. Overall, median age was 10.1 ± 3.0 years (range: 3 to 17), 51 % were female. An average of 3.6 patients/year (range: 0 to 9) was diagnosed, 67.3 % of patients presented in the last 15 years. Forty-nine percent of patients were Canadian-born non Aboriginal (CbnA: N = 48) and the remaining were either Canadian-born Aboriginal (CbA: N = 11; 11.2 %) or Foreign-born (Fb: N = 39; 39.8 %). The median rate of ARF in Montréal was calculated at 1.2/100,000, for the total length of observation: CbnA 0.8/100,000, CbA 10.7/ 100,000 and Fb 2.1/100,000. A positive family history of ARF was known in only three patients.
Clinical presentation
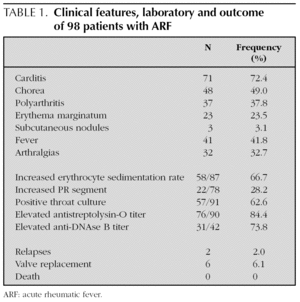
Carditis was the most frequent clinical manifestation of ARF (72.4 %) followed by Sydenham chorea (49.0 %) (table 1). In our cohort, 51.6 % patients (n = 47) had simultaneously a positive throat culture and elevated ASO at the time of presentation.
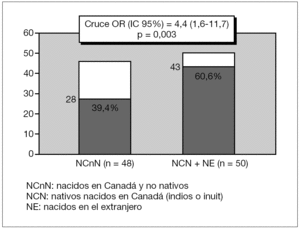
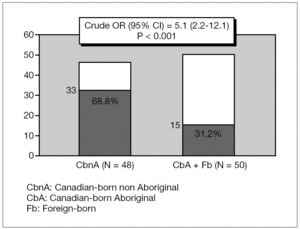
CbA or Fb children had more than four-fold increased risk of carditis compared to CbnA children, Crude OR (95 %IC) 4.4 [1.6-11.7] p = 0.003 (fig. 1). CbnA children presented more than five-fold increased risk of chorea compared to children from other cultural backgrounds, Crude OR (95 %IC) 5.1 [2.2-12.1] p < 0.001 (fig. 2).
Figure 1. Distribution of cardiac manifestations (N = 71) according to the ethnic origin.
Figure 2. Distribution of chorea (N = 48) according to the ethnic origin.
Among 71 patients with carditis diagnosed clinically or by echocardiography, 16 patients (16.3 %) had congestive heart failure and 31 children had involvement of more than one valve. The association of mitral and aortic regurgitation was found in 40.8 % of patients (n = 29), isolated mitral valve or aortic regurgitation were found respectively in 46.5 % and 9.9 % patients (n = 33 and 7); mitral stenosis in 2.8 % (n = 2) and aortic stenosis in 1.0 % (n = 1). Five patients presented with pericarditis and in three children, cardiac nodules were described at the echocardiography.
Forty-eight out of 98 children (49 %) presented with the manifestation of chorea. It was seen significantly more frequently in females (32/48 = 66.7 %) than in males: Crude OR (95 %IC) = 3.6 [1.5-8.2], p = 0.003. Isolated chorea occurred in 29.2 % patients (n = 14); and was unilateral in 43.8 % children (n = 21). Imaging abnormalities at computed tomography or MRI of the brain were found in 44.8 % patients (13 over 29). The mean duration of chorea was 4 months (range: 1-24). Eight patients were treated with Haloperidol, 13 with Valproic Acid, 12 received others drugs and 15 received no treatment. Three patients suffered a prolonged dysarthria with complete resolution after speech therapy.
Isolated polyarthritis, as the only major criteria was observed in 16.3 % patients (n = 16). Erythema marginatum and subcutaneous nodules were found in 23.5 % and 3.1 % of children, respectively. Both carditis and polyarthritis were found in 28.6 % patients (n = 28); carditis and chorea in 30.6 % patients (n = 30), polyarthritis and chorea were presented in two patients (2.0 %) and the three major criteria of polyarthritis, carditis and chorea in two others patients (2.0 %).
By the time this review was done: 2 patients suffered clinical relapses; 6 patients (6.1 %) required valve replacements, 4 of them in the five years following the initial diagnosis; no deaths were attributed to ARF.
Discussion
Despite a high prevalence in the third world, ARF has become a rare disease in industrialized countries, with the overall incidence which has decreased significantly to less than 1/100,000 around the world10. During the last decades, ARF has also been on the decline in Canada; the Toronto ARF11 registry reported in 1961 an incidence of 210/100,000, and in the late 1980s of 0.96/100,000. Our recent experience shows an overall median rate of ARF in Montréal of 1.2/100,000.
Certain ethnic groups may be more susceptible to ARF than others2,12-14. In 1982, Longstaffe15, in Manitoba, Canada, reported an incidence rate of 126/100,000 in native and 29/100,000 in non-native children. In our series in Montreal, we found an incidence for CbnA of 0.8/100,000 compared to 10.7/100,000 for CbA and 2.1/100,000 for Fb. The reasons for this observation have not yet been established, since data available were not sufficient to conclude that overcrowding or any other specific factor was responsible. Although, we believe that social and economic factors and in particular housing conditions, are the major reasons for the high rates found in these specifics populations. Given the relatively small native population in Quebec [n = 3814 children over total population of 293,350 of children 5-19 years old (statistics from 2001)], it appears that this ethnic group is at higher risk. This may be due to high crude colonization rate of Streptococcus16,17, genetic predisposition18, poor socio-economic conditions, overcrowding and diminished access to primary health care19.
Specific HLA antigens and different alloantigen expressed at the surface of lymphocytes and recognized by monoclonal antibodies appear to be a markers of susceptibility to RF and expressed only after stimulation by specific rheumatogenic strains of group A Streptococcus20,21. The high prevalence of Streptococci among Inuit children has been published previously22. In our series, information according to family income or persons/household was not available. The decreased incidence of ARF in the world, has been attributed to improved living conditions and medical care, the introduction of antibiotics and the changes in prevalence of rheumatogenic group A Streptococcus23.
During the study period, we have seen an average of 3.5 new cases of ARF/year without significant outbreaks such as reported in the United States2 or in other provinces of Canada24,25. Instead, we calculated 2.7 cases/year in the period 1979-1984, 2.8 cases/year in 1985-1994 and 4.9 cases/year in 1995-2005. It is even possible that the real incidence of ARF in our environment might be higher than the rate observed because we have only included hospitalized patients with diagnostic of ARF; thus the incidence of ARF may have been underestimated if patients were managed without hospitalization26. The likelihood of children hospitalized in other secondary care centers surrounding Montreal is not likely, as they reported having not seen patients with ARF for a very long time (personal communication).
Carditis was diagnosed in 72.4 % of our patients; a similar incidence was found in US in the 1980's27. As known from the literature, carditis is diagnosed in 50 % of cases on clinical examination and 70 % by cardiac sonography28. In our cohort, carditis was statistically significant more commonly found among CbA or Fb than in CbnA. In industrialized countries, long-term prognosis of carditis has improved because the disease itself seems to have a modified course and also because antibiotic prophylaxis has prevented subsequent attacks4. We outline the good prognosis of our cohort with only two cases of relapse and only 6.1 % of patients requiring valve replacements, both statistics comparing favourably to other series. This could have been related to a good compliance to prophylaxis combined with a decreased exposure to other rheumatogenic group A Streptococcus strains29.
In our series, chorea was present in 49.0 %. In the 1990's, Allen30 had published an incidence of chorea associated with ARF of only 6 % in Toronto, Canada. Our incidence of chorea was also higher than the 6-31 % reported in the literature2,27,31-34. Patients who present with Sydenham's chorea often lack evidence for Group A streptococcal infection34. Nevertheless, in our patients with chorea, 60 % had a positive throat culture and 52 % had an elevated ASO titer. Zomorrodi33, in Pennsylvania, US, confirmed streptococcal infection in 99 % of 71 patients with chorea. One important finding from our series was that chorea was statistically more commonly found in CbnA than in children from other cultural backgrounds. Pathogenesis of Sydenham's chorea relates to a genetic susceptibility or different virulence factors of rhumatogenic streptococcal strains31,34. However, little work has been done on the microbiological characteristics of the particular group A streptococcal strains which modulate the clinical expression of the disease. In our study, we did not include any case of PANDAS (The Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infection)35.
We found that polyarthritis was present in 37.8 % of patients, less frequently than the 50-80 % reported in the literature27,36. Isolated polyarthritis, as a single major criterion of ARF, associated with fever and increased acute-phase reactants is the weakest diagnostic criteria of ARF and a problem clinician's may encounter in practice; in our cohort, it was accepted in 16 patients.
Documentation of an antecedent streptococcal infection is required for diagnosis of ARF7. In our cohort, 62.6 % of patients tested at the time of diagnosis had positive throat culture for Group A streptococcus. These results have limited usefulness, because it neither distinguishes between recent infection and a more long standing pharyngeal carriage37. Nevertheless, this result was confirmed in our cohort, as we found elevated ASO and anti-DNase B titer in 84.4 % and 73.8 %, respectively and a positive throat cultures was simultaneously associated with elevated ASO in 51.6 % of patients. Specific serotypes of GAS have been associated with the development of ARF2,5. Unfortunately the serotypes of the streptococci were unavailable in our review.
Although our study involves a large series of ARF in children from Montreal, its retrospective nature and the evaluation of only hospitalized cases in tertiary health centers constitute limitation factors. Rheumatic fever may have different clinical presentations in developing countries according to genetic predisposition, prevalence of rheumatogenic strains and socio economical conditions. The results of the present study reflect the clinical picture of an industrialized North American city.
In conclusion, although, it is important to emphasize that ARF has become a rare disease in industrialized countries, it remains prevalent in our environment and clinical presentation varied depending on the ethnicity. The most striking fact in our study was that chorea was significantly more frequently observed in Canadian born-non aboriginal children. Further studies are needed to identify the virulent factors and epitopes of rheumatogenic streptococcal strains as well as the genetic markers of predisposition to explain the differences in this clinical presentation. Physicians ought to know this pathology, in order to recognize and diagnose correctly children with episodes of ARF, a disease which is still with us.
Corresponding author: Ana Carceller MD.
Department of Pediatrics.
CHU Sainte-Justine.
3175 Côte Ste-Catherine.
Montréal. Québec H3T 1C5. Canada.
E-mail: ana_carceller@ssss.gouv.qc.ca