To analyse the impact of a multidisciplinary and decentralised safety committee in the paediatric management unit, and the joint implementation of a computing network application for reporting medication errors, monitoring the follow-up of the errors, and an analysis of the improvements introduced.
Materials and methodsAn observational, descriptive, cross-sectional, pre-post intervention study was performed. An analysis was made of medication errors reported to the central safety committee in the twelve months prior to introduction, and those reported to the decentralised safety committee in the management unit in the nine months after implementation, using the computer application, and the strategies generated by the analysis of reported errors.
Measured variablesNumber of reported errors/10,000 days of stay, number of reported errors with harm per 10,000 days of stay, types of error, categories based on severity, stage of the process, and groups involved in the notification of medication errors.
ResultsReported medication errors increased 4.6-fold, from 7.6 notifications of medication errors per 10,000 days of stay in the pre-intervention period to 36 in the post-intervention, rate ratio 0.21 (95% CI; 0.11–0.39) (P<.001). The medication errors with harm or requiring monitoring reported per 10,000 days of stay, was virtually unchanged from one period to the other ratio rate 0.77 (95% IC; 0.31–1.91) (P>.05). The notification of potential errors or errors without harm per 10,000 days of stay increased 17.4-fold (rate ratio 0.005, 95% CI; 0.001–0.026, P<.001).
ConclusionsThe increase in medication errors notified in the post-intervention period is a reflection of an increase in the motivation of health professionals to report errors through this new method.
Analizar el impacto en la notificación de errores de medicación de la puesta en marcha de un comité de seguridad multidisciplinar descentralizado en la unidad de gestión pediátrica, e implantación conjunta de una aplicación informática en red para la comunicación de errores de medicación, mediante seguimiento de los errores y análisis de las mejoras.
Material y métodosEstudio observacional, descriptivo, transversal pre-post intervención. Se analizan los errores de medicación notificados a la comisión central de seguridad, en los 12 meses previos a la implantación, y los notificados mediante la aplicación informática descentralizada a la comisión de seguridad de la unidad de gestión, en los 9 meses posteriores, y las estrategias generadas por el análisis.
Variables medidasNúmero de errores notificados por 10.000 días de estancia, número de errores con daño por 10.000 días de estancia, tipo, categoría en función de la gravedad, fase del proceso, colectivo que notifica y medicamentos implicados.
ResultadosSe multiplican por 4,6 los errores de medicación notificados —7,6 notificaciones por 10.000 días de estancia en el periodo preintervención y 36 en el postintervención—, razón de tasas de 0,21 (IC 95%: 0,11–0,39) p<0,001.
No cambian prácticamente los errores con daño o que necesitaron monitorización notificados por 10.000 días de estancia de un periodo a otro, razón de tasas: 0,77 (IC95%: 0,31–1,91) p>0,05. Se multiplica por 17,4 la notificación de errores sin daño o potenciales por 10.000 días de estancia, razón de tasas: 0,005 (IC 95%: 0,001–0,026) p<0,001.
ConclusionesEl incremento de los errores de medicación notificados en el periodo postintervención es reflejo del aumento en la motivación de los profesionales sanitarios para notificar a través de este nuevo método.
An adverse drug event1,2 (ADE) is defined as any harm, severe or mild, caused by the medical use of a drug. ADEs are classified into: (1) medication errors (MEs): any preventable incident that may harm the patient or result in the inappropriate use of a drug; and (2) adverse drug reactions (ADRs): an effect which is noxious and unintended, usually nonpreventable, that occurs after the administration of a drug. ADRs are unavoidable.
A potential adverse event, potential error, or “near miss”, is an incident that did not result in injury.
A spontaneous reporting system is a method of pharmacovigilance based on the communication, collection, and evaluation of reports of suspected ADRs. According to the law3, MEs that cause harm to the patient must be reported and will be considered as ADRs upon notification, save for errors that result from therapeutic failure due to omission of treatment.
Some of the characteristics of an effective ME notification system described in the literature are2: voluntary reporting; invites the active participation of healthcare professionals and patients; gives the choice of anonymous reporting; guarantees the confidentiality of reported information; takes a nonpunitive approach to reporting; encourages reporting of both potential and actual errors of patient injuries resulting from errors; and provides feedback of error analysis and recommendations.
Additional characteristics of a reporting system include: ease of use; availability of both electronic and paper formats; standard taxonomy; severity of outcomes; retrievable data; report generation; and root-cause analysis.
The number of errors that occur in the daily delivery of healthcare is much higher than we would think. It is estimated that 50–96% of errors go unreported. In the EMOPEM study,4 the mean error rate for the 22 participating hospitals was 21.72%, with a minimum of 2.85% and a maximum of 79.02%.
Underreporting of MEs can compromise patient safety. The perceived barriers to notification are fear of consequences; a blame culture; lack of training in reporting, time to report, organisational leadership and support, legal protection, guidelines and policies, staff and resources; lack of understanding why reporting is needed; concern that no action will follow reporting; non-anonymous reporting; and reporting perceived to be bureaucratic.5,6
Paediatric rates of potentially serious MEs can be 3 times greater than adult rates.7,8 The risk of MEs in paediatrics is particularly high because of the need for dosage calculations based on the patient's weight, age, or body surface area and the patient's condition. Unlicensed use of medications, for which there is little information on the adequate dosage, is also common (for instance, in off-label use or the treatment of rare diseases such as cystic fibrosis).
For potent drugs, when only a small fraction of the adult dose is required for children, it becomes very easy to cause errors because of miscalculation or misplacement of the decimal point.9–13 Furthermore, it is often necessary to manipulate adult formulations to obtain smaller doses for paediatric patients. These practises are associated with a high risk for errors, as the bioavailability of a drug that has been manipulated is often unknown and unpredictable. There is a lack of information on compatibility and stability.8
“High alert medications” are defined as drugs that bear a heightened risk of causing significant patient harm or even death when they are used in error. This definition does not suggest that errors associated with these drugs are necessarily more common, but that the consequences of these errors are more severe for the patients. Thus, high alert medications should be a priority objective in any hospital's clinical safety programmes.14
Systematic mechanisms to promote safe medication are probably important factors that allow the translation of a safety culture into outcomes, but they may be ineffective in the context of a poor safety culture.15
An overall organisational culture based on trust and error disclosure predicts the intent to disclose a hypothetical error in a patient, while teamwork and a safety culture do not.16
Isolated ME rates, based on incident notifications, do not provide a valid measure of patient safety. A high error rate may be suggestive of dangerous practises or of an organisational culture that promotes error reporting. A low rate of MEs could suggest that an organisation engages in successful and safe practises, or that it has a particularly punitive approach to reporting.5
Reported near misses lead to corrective action at the organisational level when managers perceive a substantial potential for harm and preventability.17
Observational studies4,18 help detect safety problems, but are very labour-intensive. The idea for this study came from an observational study that we had conducted in the paediatric oncology unit.19
To measure the safety interventions related to the use of medications we have analysed the variation in reporting rates of MEs, variation in incidence rates of MEs with harm per 10,000 distributed doses, and improvement in the responses to safety questionnaires.20,21
The incidence density of MEs is calculated as the number of errors per 100 patients per duration of hospital stay in days.18
We selected 2 factors for our intervention, one based on the culture of error disclosure, and one based on trust.16 The decentralisation of reporting and monitoring of MEs at the management level may lead to increased motivation to report in healthcare professionals. It would make sense to make it easier to measure harm, understand causes, seek solutions, implement strategies for improvement, and measure the impact of these strategies within a group of closely interacting professionals.
The 2010–2014 Plan de Calidad del Sistema Sanitario Público de Andalucía (Quality Plan of the Public Health System of Andalusia)22 contemplates the decentralisation of patient safety organisation to the management units. This inspired us to institute a safety committee in the paediatrics management unit.
The reviewed literature expresses that further research is needed to understand how the way reporting is done affects learning from errors and error prevention, and to identify factors that may lead to improved reporting.6
Our hypothesis, based on the available evidence, is that a feeling of trust towards a safety committee within the management unit and easier reporting by means of a simple computer application could contribute to the motivation to report MEs and lead to improved reporting rates.
Our objective was to analyse the impact on error notification of the implementation of a decentralised multidisciplinary safety committee in the paediatrics management unit and the concurrent introduction of a networked computer application for ME reporting by monitoring the error reports and evaluating the safety-improvement strategies implemented by the management unit.
MethodologyWe conducted an observational, descriptive, pre-post intervention study.
Description of new strategy1. A networked computer application was designed for the confidential reporting and analysis of MEs in the management unit.
Fields of an error report in the computer applicationError description, severity classification, affected organs or systems, patient age, clinical manifestations, sex, drug/active ingredient, dose, error date, day of the week, type of incident, setting where the error originated, medication process (dispensation, prescription, transcription, preparation, administration), setting where the error was detected, cause of the error, person who made the error, contributing factors, person who discovered the error, measures proposed or taken to prevent the same error from occurring.
Types of incidentWrong medication, pharmacological treatment or dose omission, wrong dose, incorrect frequency of administration, wrong dosage form, preparation error, manipulation and/or packaging errors, wrong administration technique, wrong route of administration, wrong rate of administration, wrong time of administration, wrong patient, wrong duration of treatment, insufficient treatment monitoring, deteriorated drug, patient noncompliance.1
Severity categories| Cat a: | Potential error: incident or circumstance that has the capacity to cause harm. |
| Cat b: | Error without harm: an error occurred but did not reach the patient. |
| Cat c: | Error without harm: the error reached the patient but did not cause patient harm. |
| Cat d: | Error without harm: the error reached the patient and did not cause patient harm, but required monitoring/intervention. |
| Cat e: | Error with harm: the error caused temporary harm to the patient and required intervention. |
The application was introduced to the staff in clinical meetings of the medical staff and in small group meetings (6 people) for the nursing staff. In both cases, the contents had been developed by consensus
2. A safety committee was instituted within a paediatrics management unit with the participation of clinicians, nurses, and pharmacists.
Monthly meetings lasting one hour were held the first Thursday of every month to analyse MEs and develop improvement strategies based on the analysis. Error analysis feedback was provided to the healthcare staff and the consensus improvement strategies were notified by the submission of meeting minutes to managerial staff.
Analysis of the impact of new strategy3. An audit was done to analyse MEs—those notified in paper to the central safety committee in the 12 months prior to implementation, as well as those reported by means of the decentralised computer application to the safety committee of the management unit in the 9 months after implementation—and the strategies generated by the analysis of MEs in the pre- and postintervention periods.
Measured variables: number of reported errors per 10,000 days of hospitalisation, overall for each period and by month; number of errors with harm per 10,000 days of hospitalisation; type; severity category; step of the process; professional category of reporter; and medication involved.
The OpenEpi®23 application was used to do the statistical analysis. We performed a descriptive analysis of the variables, calculating the various relative frequencies.
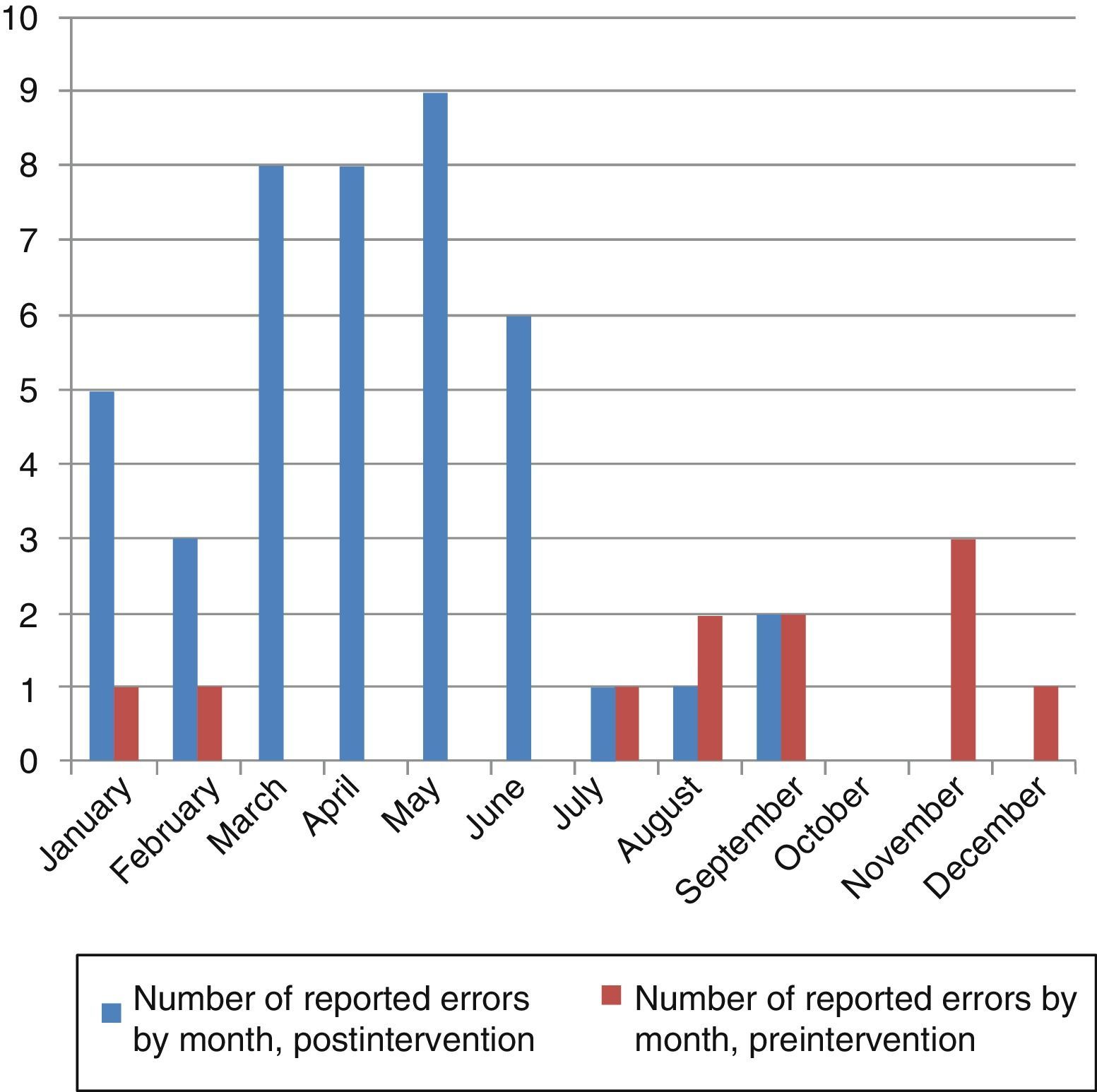
ResultsPreintervention period (January–December 2011): 13 error reports/17,124 days of hospitalisation (7.5/10,000) in children aged 4±4 years; mean monthly number of reports±standard deviation (SD): 1±1.
Number of errors with harm or which needed monitoring reported per 10,000 days of hospitalisation: 2.9.
Postintervention period (January–September 2012): 42 error reports/11,801 days of hospitalisation (36/10,000), in children aged 5±4 years; mean monthly number of reports±SD: 5±3.
Number of errors with harm or which required monitoring reported per 10,000 days of hospitalisation: 3.4.
There was a 4.6-fold increase in the number of ME reports/10,000 days of hospitalisation in the postintervention period relative to the preintervention period. When we compared the rate of error reporting in the 2 periods, we obtained a rate ratio of 0.21 (95% CI, 0.11–0.39) P<.001.
The total number of errors with harm that required reporting per 10,000 days of hospitalisation hardly changed between periods (rate ratio, 0.77; 95% CI, 0.31–1.91; P>.05).
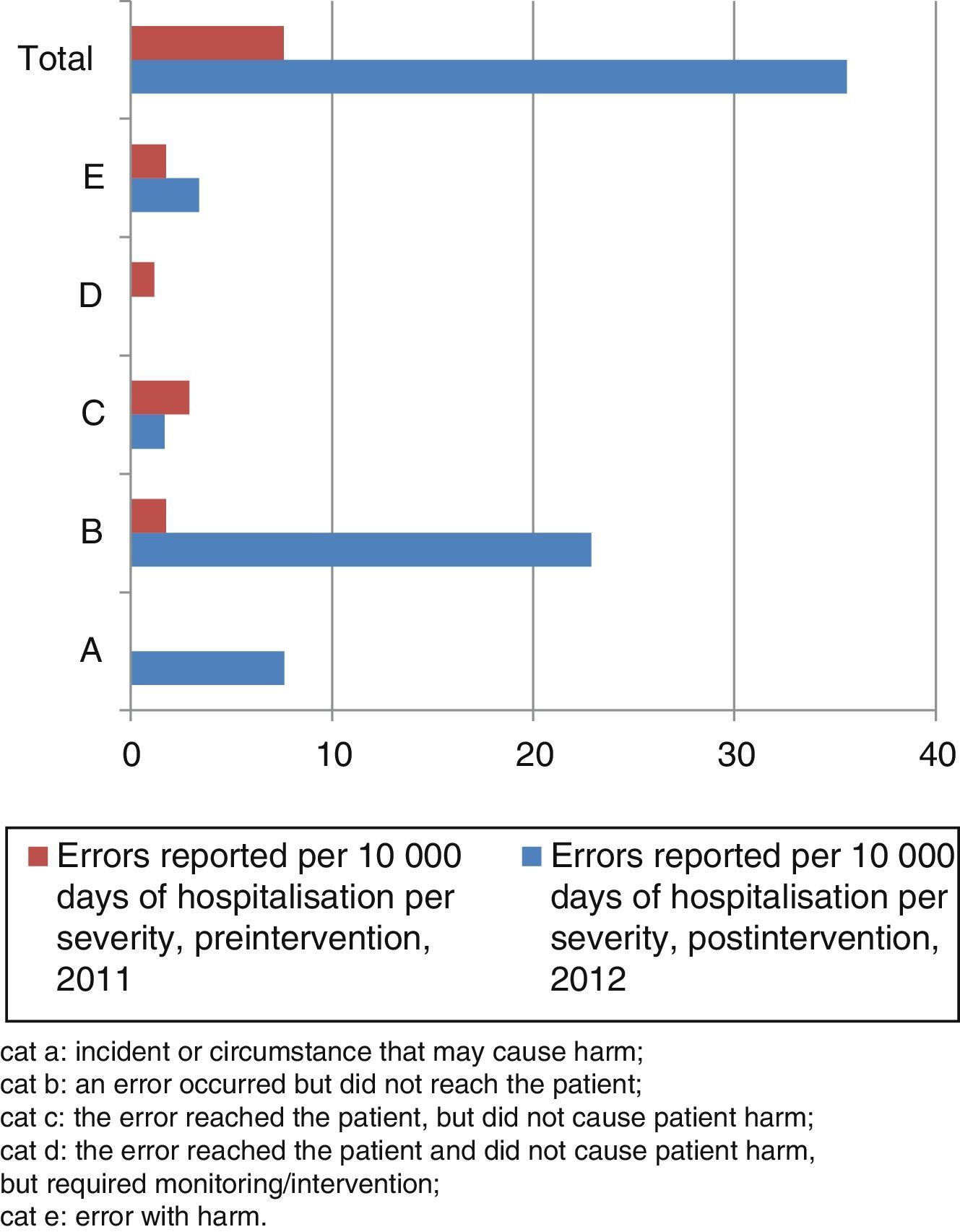
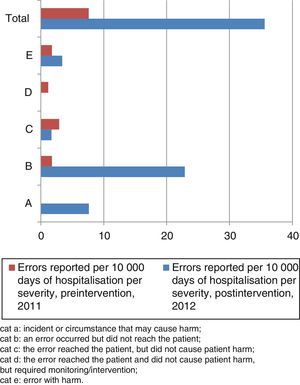
The severity categories for the MEs per 10,000 days of hospitalisation reported in the pre- and postintervention periods are shown in Fig. 1.
Medication error categories by severity. Cat A: incident or circumstance that may cause harm; cat B: an error occurred but did not reach the patient; cat C: the error reached the patient, but did not cause patient harm; cat D: the error reached the patient and did not cause patient harm, but required monitoring/intervention; cat E: error with harm.
The reported number of potential errors and errors without harm per 10,000 days of hospitalisation increased by a factor of 17.4 in the postintervention period relative to the preintervention period (rate ratios, 0.005; 95% CI, 0.001–0.026; P<.001).
In the preintervention period, all the reported errors with harm occurred during administration. In the postintervention period, 75% corresponded to prescription errors and 25% to administration errors.
In the preintervention period, the errors with harm, all of them related to the process of administration, were: morphine hydrochloride overdose, vancomycin extravasation and intrathecal methotrexate overdose. The errors that required monitoring were administration of a NSAID to an allergic patient and of an excess dose of phenobarbital.
The errors with harm in the postintervention period were pain during infusion of paracetamol (administration), prescription of an overdosage of dactinomycin, anaphylactic shock secondary to metamizol administration, and prescription error consisting of swapping the doses of 2 antibiotics.
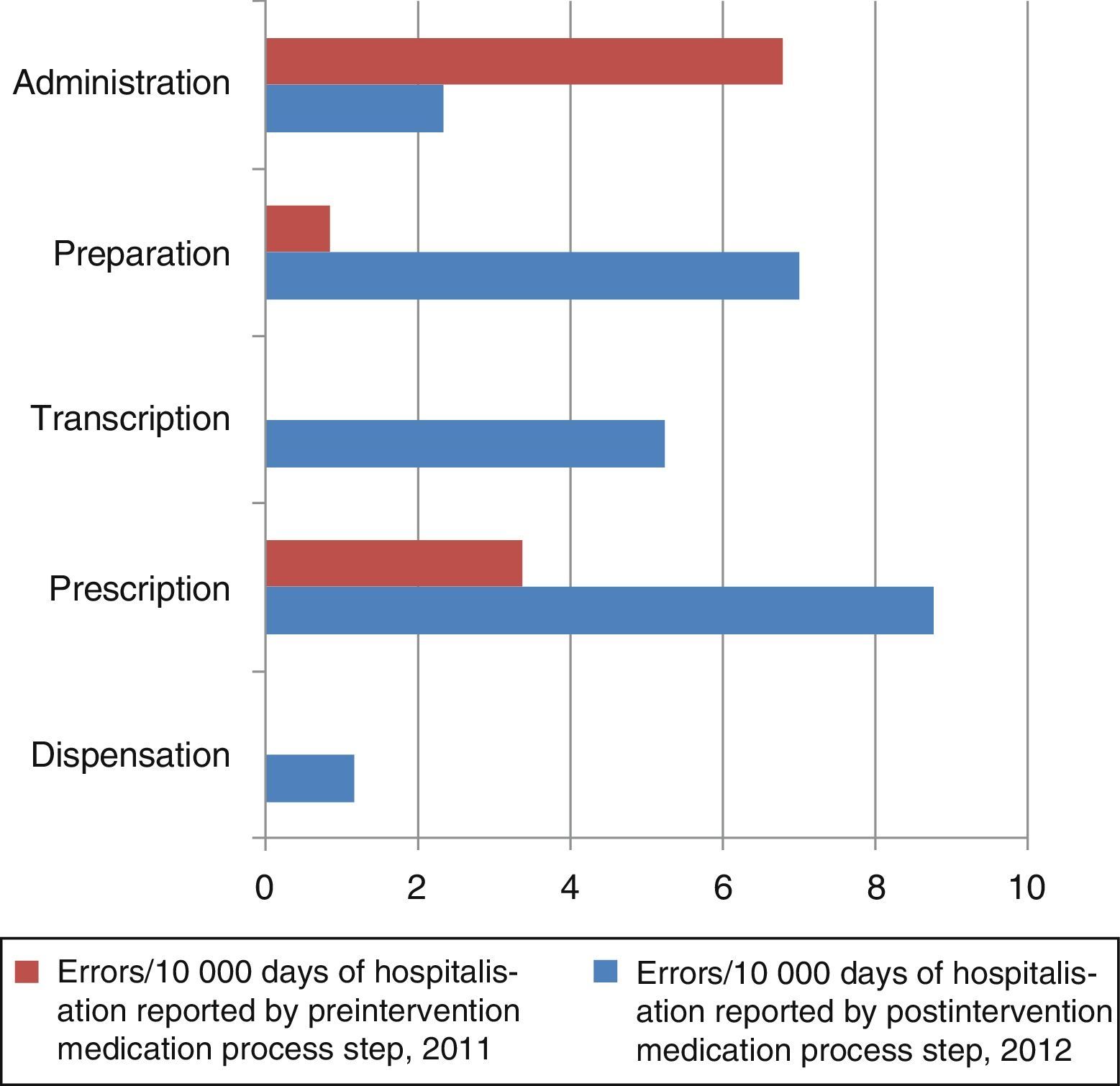
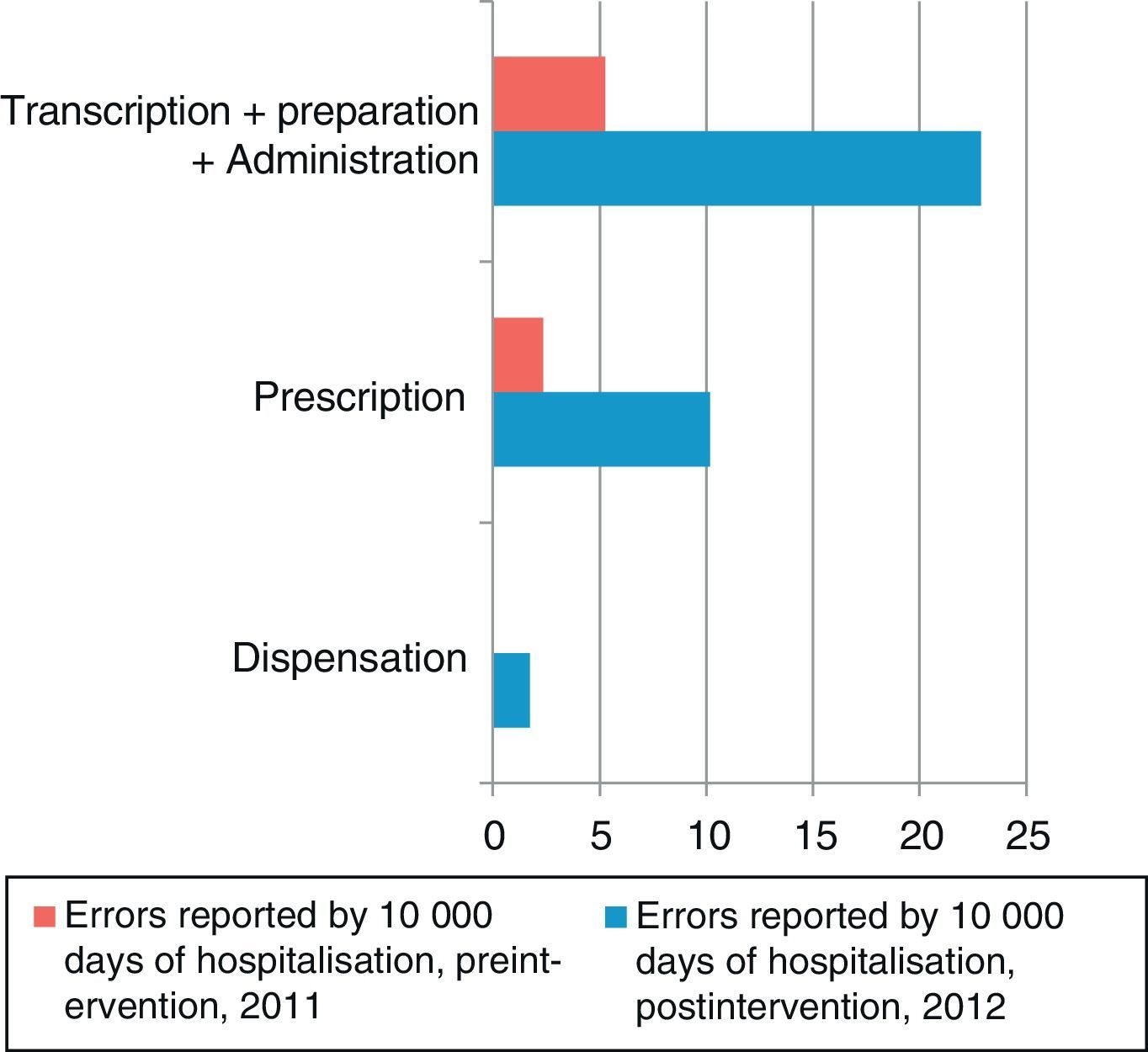
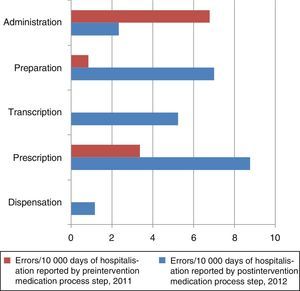
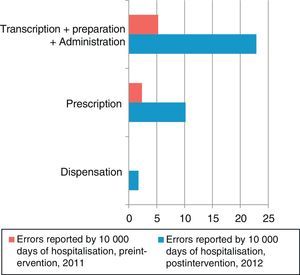
The number of MEs per 10,000 days of hospitalisation according to the step in the medication process is shown in Fig. 2. The steps carried out by the nursing staff (transcription, preparation, and administration) and by clinicians (prescription) are grouped together in Fig. 3. In the preintervention period, paper reports did not need to specify the steps of the process following the format used later in the postintervention period.
In the preintervention period, 100% of the errors were reported by the nursing staff; in the postintervention period, 79% were reported by nurses, 7% by physicians, and 14% by pharmacists.
The rate of reported errors per 10,000 days of hospitalisation in the steps carried out by the nursing staff increased by a factor of 3.7 (rate ratio, 0.026; 95% CI, 0.01–0.57; P<.001), and in the steps carried out by physicians, it increased by a factor of 5.4 (rate ratio, 0.018; 95% CI, 0.004–0.074; P<.001).
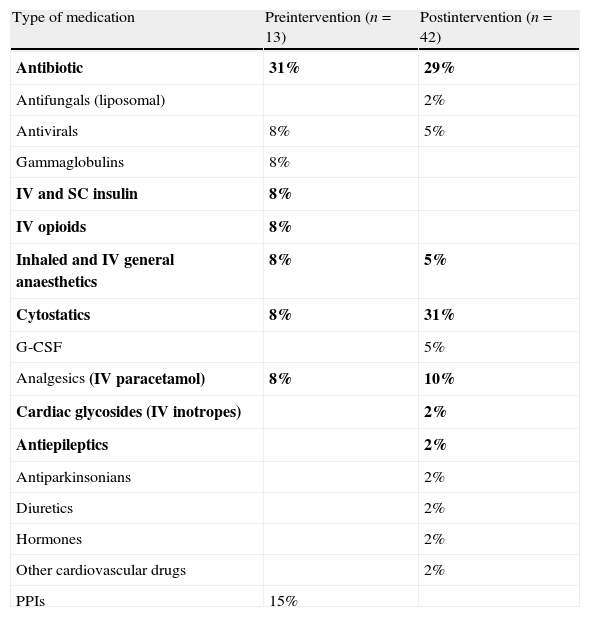
Table 1 shows the therapeutic classification of the drugs involved in the reports. We performed this classification based on the list proposed by our working group after the paediatrics management unit reached a consensus following a review of the literature.14,24
Medications involved in reports.
| Type of medication | Preintervention (n=13) | Postintervention (n=42) |
| Antibiotic | 31% | 29% |
| Antifungals (liposomal) | 2% | |
| Antivirals | 8% | 5% |
| Gammaglobulins | 8% | |
| IV and SC insulin | 8% | |
| IV opioids | 8% | |
| Inhaled and IV general anaesthetics | 8% | 5% |
| Cytostatics | 8% | 31% |
| G-CSF | 5% | |
| Analgesics (IV paracetamol) | 8% | 10% |
| Cardiac glycosides (IV inotropes) | 2% | |
| Antiepileptics | 2% | |
| Antiparkinsonians | 2% | |
| Diuretics | 2% | |
| Hormones | 2% | |
| Other cardiovascular drugs | 2% | |
| PPIs | 15% |
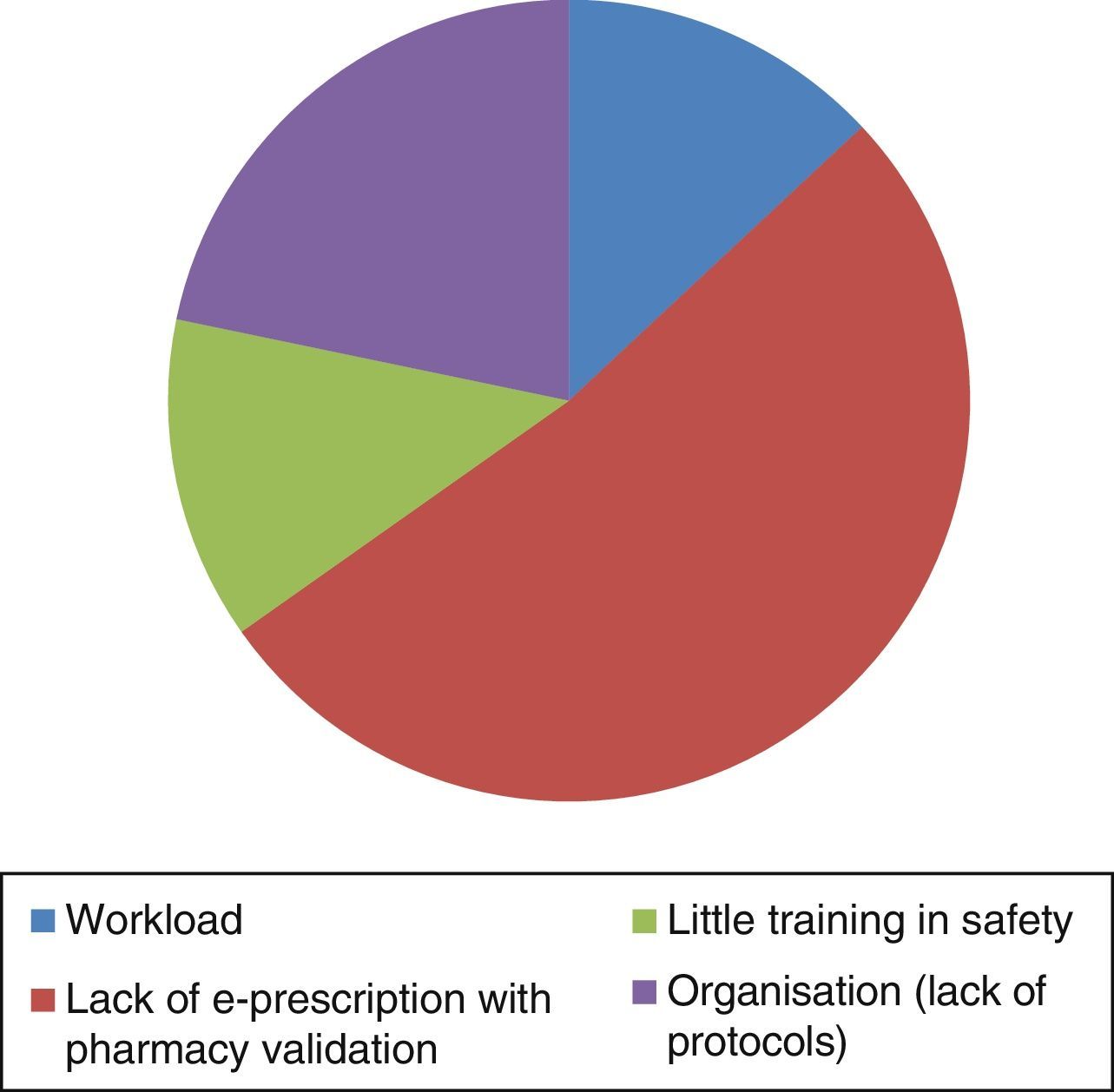
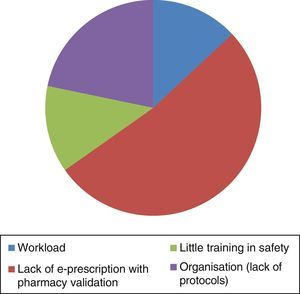
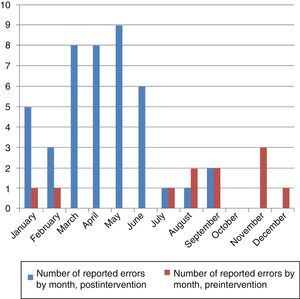
Fig. 4 shows the reported causes of MEs, and Fig. 5 the number of errors per month notified in the pre- and postintervention periods.
DiscussionThe interventions to promote a culture of safety included multicomponent strategies, with the formation of teams and mechanisms to support communication. Assessing the implemented strategies based on the outcomes was considered of paramount importance.25
In calculating reporting rates, we have used 10,000 days of hospitalisation as the denominator, rather than the 100 commonly used in observational study. We did this because the direct observation method is about 1000 times more efficacious than the method of voluntary reporting, although it tends to miss errors with harm.5,21
The substantial increase in reporting unaccompanied by an increase in reports of error with harm implies that the motivation to report of healthcare professionals has increased, as manifested by the 17-fold increase in the reporting of potential errors and errors without harm.
The involvement of nursing staff in reporting has increased, as has that of physicians and pharmacists. We were not able to assess the safety climate by means of a survey, as recommended by the Ministerio de Sanidad y Consumo (the Ministry of Health and Consumers),26 nor the training in safety. The method of training in small groups applied to the nursing staff was more effective than training during clinical sessions, as nurses have reported errors 11 times more often than specialist physicians, who are the smallest professional collective.
Consistent with what we found in our study, communications in recent national congresses also mention the lack of electronic prescription support as an important cause of MEs (44%).27
The following measures, among others, are recommended to evaluate the efficacy of patient safety strategies: account of the theoretical model explaining why the safety intervention would work, detailed description of the intervention so it can be repeated, description of how the intervention changes over time, and assessment of the effect of the intervention on outcomes.28 We followed these steps in our study. A drop in reporting was observed starting on the seventh month, which coincided with the summer. Thus, we propose refresher training on safety and ME reporting every 6 months.
We believe that the system used in the study, based on user-friendly software, confidential and nonpunitive reporting, training on error reporting based on presentations developed by consensus, and a safety committee within the unit that provides feedback, is successful in fighting the barriers to reporting described in the literature.4,6,11 In the past, the management unit had not been working in depth towards achieving an error culture and an error disclosure culture.
During the postintervention period, several strategies were proposed in the meetings of the safety committee of the management unit, which were partially implemented: (1) double-checking of paediatric chemotherapy prescriptions; (2) double-checking of high alert drug preparation; (3) introduction of an electronic form for drug prescription and administration; (4) multidisciplinary development of a manual for the administration of high alert drugs in paediatrics (in press) and (5) divulgation of the directives for the prescription and administration of drugs.
The development of interventions, feedback, and the implemented strategies involved the collaboration of clinical, nursing, and pharmacy staff at all times.
The programme described here has been implemented at no cost, as the training sessions have been integrated in previously established programmes, and the safety committee operates during regular working hours. Also, when it is implemented in a new unit, the central safety committee of the hospital is granted access to the computer application, so that experiences in improvement can be shared.
In the future, the safety committee of the unit will develop training sessions to refresh and update theoretical knowledge and techniques for the improvement of information divulgation. The manual for the use of high alert drugs in paediatric care, already developed, will be circulated, and we will evaluate the safety climate and knowledge of the different professional collectives before and after these interventions.
Conclusions- -
All professional collectives became involved during the postintervention period.
- -
The motivation of healthcare professionals to report has increased, as evinced by the considerable increase in the reporting of potential errors. The reporting of errors with harm or errors requiring monitoring hardly increased in the same period.
- -
The monthly analysis of the causes of errors in the committee is quick and effective, and is manifested in solutions that are implemented on the go and studied for feedback in short intervals of time.
- -
All of the above suggests that this decentralised reporting system is ideal to plan and monitor safety interventions in the management unit.
- -
We need to evolve at the structural and procedural levels (electronic prescription validated by the pharmacy); offering the necessary safety training; reassessing work loads; and considering the protocols needed in the unit.
- -
We need to maintain the staff's awareness of this subject by means of periodic refresher education, as the interest in reporting declines with the passing of time.
The authors have no conflicts of interest to declare.
We wish to thank all the members of the paediatrics safety committee of the Hospital Universitario Virgen del Rocío for their invaluable technical help and Mercedes Fernández Arévalo for her support.
Please cite this article as: Guerrero-Aznar MD, Jiménez-Mesa E, Cotrina-Luque J, Villalba-Moreno A, Cumplido-Corbacho R, Fernández-Fernández L. Validación en pediatría de un método para notificación y seguimiento de errores de medicación. An Pediatr (Barc). 2014;81:360–367.