Meningococcal invasive disease, including the main clinical presentation forms (sepsis and meningitis), is a severe and potentially lethal infection caused by different serogroups of Neisseria meningitidis. Meningococcal serogroup B is the most prevalent in Europe. Most cases occur in children, with a mortality rate of 10% and a risk of permanent sequelae of 20–30% among survivors.
The highest incidence and case fatality rates are observed in healthy children under 2–3 years old, followed by adolescents, although it can occur at any age.
With the arrival in Spain of the only available vaccine against meningococcus B, the Advisory Committee on Vaccines of the Spanish Association of Paediatrics has analysed its preventive potential in detail, as well as its peculiar administrative situation in Spain.
The purpose of this document is to publish the statement of the Committee as regards this vaccination and the access to it by the Spanish population, taking into account that it has been only authorised for people at risk. The vaccine is available free in the rest of Europe for those who want to acquire it, and in some countries and regions it has been introduced into the systematic immunisation schedules.
The Committee considers that Bexsero® has a profile of a vaccine to be included in the official schedules of all the Spanish autonomous communities and insists on the need for it to be available in pharmacies for its administration in all children older than 2 months.
La enfermedad meningocócica invasora, con sus 2 formas de presentación principales (sepsis y meningitis), es una patología grave y potencialmente mortal, causada por distintos serogrupos de Neisseria meningitidis, entre los cuales, actualmente, predomina el serogrupo B en Europa. La mayoría de los casos se producen en la edad pediátrica, con una mortalidad aproximada del 10% y un riesgo de secuelas permanentes del 20-30% entre los supervivientes. Presenta mayor incidencia y letalidad en niños sanos menores de 2-3 años, seguidos de los adolescentes, aunque puede ocurrir a cualquier edad.
Hasta ahora no se contaba con ningún arma inmunopreventiva contra el meningococo B. Así, con la llegada a España de la única vacuna actualmente disponible, el Comité Asesor de Vacunas de la Asociación Española de Pediatría (CAV-AEP) ha analizado detalladamente tanto el potencial preventivo de dicha vacuna, como la situación peculiar administrativa de la misma en España. El objetivo de este documento es informar del posicionamiento del CAV-AEP en relación con la vacuna frente al meningococo B y el acceso a la misma por parte de la población infantil española, teniendo en cuenta que ha sido autorizada exclusivamente para el uso hospitalario en personas de riesgo. En Europa, la vacuna sí está disponible en farmacias, incluso incluida en calendarios oficiales de algunos países o regiones.
Este comité considera que Bexsero® presenta un perfil de vacuna a incluir en todos los calendarios españoles y que debería estar disponible libremente en farmacias para su administración en todos los niños mayores de 2 meses.
Invasive meningococcal disease (IMD) is a severe and potentially fatal pathology caused by various Neisseria meningitidis serogroups. Currently, the most prevalent type in Spain and the rest of Europe is serogroup B. The disease primarily affects children younger than 2 or 3 years, and until now we lacked an immunopreventive strategy to fight it.
With the arrival in Spain of the only currently available vaccine against serogroup B meningococcus (MenB), the Comité Asesor de Vacunas de la Asociación Española de Pediatría (Advisory Committee on Vaccines of the Spanish Association of Paediatrics [CAV-AEP]) has analysed its preventive potential and seeks to present the peculiar administrative situation of the vaccine in Spain and the stance of the committee on this status quo, in order to offer recommendations for the paediatric population residing in Spain.1
The arrival of new vaccines, such as this one, to the dismal arena of the present public health field in Spain, is complex.2 While the vaccine is approved and available to anyone who wishes to gain protection against the disease in all other European countries,3 and is even included in the official routine immunisation schedules of some countries or regions, in Spain the Ministry of Health not only does not recommend its inclusion in the official schedule in line with a document by the working group of the Ponencia y Registro de Vacunaciones (Vaccination Communications and Registry),4 but furthermore, through the Agencia Española de Medicamentos y Productos Sanitarios (Spanish Agency of Medicines and Health Products [AEMPS]),5 it has restricted its availability to hospital pharmacies, so that only a very small number of individuals may legally have access to it.
Thus, doctors in Spain are prevented from prescribing a medicine, in this case a vaccine, approved by the official competent European institution, the European Medicines Agency (EMA), for its use in any individual older than 2 months.3 This committee, along with many healthcare professionals in and outside of Spain, believes that the decision of not including the vaccine in routine schedules should not be tied to the decision to allow individuals to exercise their right to protect themselves.6
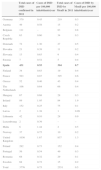
Burden of meningococcal B diseaseAccording to data from the European Centre for Disease Prevention and Control, the incidence of serogroup B meningococcal disease in Europe was of 0.77 cases per 100,000 inhabitants per year in 2011, ranging from 0.09 (Latvia) to 1.99 (Ireland) cases per 100,000 inhabitants (Table 1). At present, due to the routine use of the vaccine against serogroup C meningococcus, the number of cases due to this group has declined dramatically. As a result, serogroup B is the most prevalent in Europe (73.6%).7 At any rate, serogroup B tends to cause epidemic waves over long cycles, and we are currently witnessing the lowest incidence in the past 20 years. The number of cases in 2011 had declined by 20% compared to 2008.7
Epidemiology of invasive meningococcal disease (overall and caused by serogroup B) in the European Union in year 2011.7,9
| Total cases of IMD confirmed in 2011 | Cases of IMD per 100,000 inhabitants/year | Total cases of IMD by MenB in 2011 | Cases of IMD by MenB per 100,000 inhabitants/year | |
|---|---|---|---|---|
| Germany | 370 | 0.45 | 219 | 0.3 |
| Austria | 49 | 0.58 | 15 | 0.2 |
| Belgium | 111 | – | 83 | 0.8 |
| Czech Republic | 63 | 0.60 | 34 | 0.3 |
| Denmark | 72 | 1.30 | 27 | 0.5 |
| Slovakia | 21 | 0.39 | 11 | 0.2 |
| Slovenia | 13 | 0.63 | 9 | 0.4 |
| Estonia | 7 | 0.52 | 5 | 0.4 |
| Spain | 431 | 0.93 | 304 | 0.7 |
| Finland | 34 | 0.63 | 19 | 0.4 |
| France | 563 | 0.87 | 395 | 0.6 |
| Greece | 52 | 0.46 | 43 | 0.4 |
| The Netherlands | 106 | 0.64 | 66 | 0.4 |
| Hungary | 67 | 0.68 | 28 | 0.3 |
| Ireland | 89 | 1.95 | 84 | 1.9 |
| Italy | 152 | 0.25 | 75 | 0.1 |
| Latvia | 2 | 1.38 | 1 | 0.09 |
| Lithuania | 42 | 0.10 | 28 | 0.9 |
| Luxembourg | 2 | 0.39 | – | – |
| Malta | 6 | 1.44 | 2 | 0.5 |
| Norway | 37 | 0.75 | 10 | 0.2 |
| United Kingdom | 1036 | 1.67 | 837 | 1.3 |
| Poland | 282 | 0.73 | 152 | 0.4 |
| Portugal | 56 | 0.54 | 49 | 0.3 |
| Romania | 68 | 0.32 | 29 | 0.1 |
| Sweden | 68 | 0.72 | 15 | 0.2 |
| Total | 3776 | 0.75 | 2551 | 0.5 |
ECDC, European Centre for Disease Prevention and Control; IMD, invasive meningococcal disease; MenB, serogroup B meningococcus.
The incidence of meningococcal disease varies with age, with the highest rates found in infants, followed by adolescents and young adults. According to data from 2011, in Europe the incidence in infants was of 12.3 cases per 100,000 inhabitants, followed by that of children aged 1–4 years (4.1 per 100,000) and adolescents and young adults 15–24 years of age (1.3 per 100,000).7
Burden of meningococcal disease in SpainAccording to data published in the Boletín Epidemiológico Semanal, for the 2006–2007 period, the incidence rate of reported IMD cases (confirmed cases and suspected but unconfirmed cases) was 1.37 per 100,000 inhabitants, decreasing to 1.21 per 100,000 inhabitants in 20108 and to 0.93 per 100,000 in 2011.9
For serogroup B, the incidence was 0.77 per 100,000 inhabitants per year.7 The highest rates corresponded to children younger than 5 years (13 cases per 100,000 for infants younger than one year, and 4.3 cases per 100,000 inhabitants in the 1-to-4 year old age group).10
The incidence of IMD by serogroup B in Spain is slightly higher than the European mean, ranking fifth after Ireland, United Kingdom, Lithuania and Belgium in the European Union (EU), and second among the most populous countries.7 In terms of the total number of cases, Spain ranks third in Europe after the United Kingdom and France.7
The decline in the number of cases of serogroup B disease observed in recent years is similar to that seen in other European countries.8 Its cyclical nature requires strict surveillance in order to generate data towards the potential use of vaccines with different formulations, and to monitor the impact of the use of these vaccines. For example, in the 1975–1985 decade, meningococcal disease in Galicia reached rates of 30 cases per 100,000 inhabitants.8
Consequences of serogroup B meningococcal diseaseIt is estimated that between 10% and 14% of cases of IMD are fatal, and that between 8% and 20% to 30% of survivors suffer from long-term sequelae.11,12 In 2011, the mortality rate in Spain reached 13.6% (95% CI, 10.5%–17.2%), somewhat above the European mean (8.7%; 95% CI, 7.7%–9.6%), and was very similar across all age groups.7 The mortality was slightly lower in cases caused by serogroup B, but this must be interpreted with caution considering the low number of cases that are due to other individual serogroups.7
Some of the sequels associated to meningitis or meningococcal septicaemia are hearing loss, amputations, skin complications, psychosocial problems, hydrocephalus, other neurological and developmental disorders, and kidney failure.11,13
It is important that we consider the economic impact and the burden of hospitalisation that result from IMD in Spain. A study on the hospitalisations and deaths associated with IMD based on data from the Conjunto Mínimo Básico de Datos (mandatory basic set of data documented at discharge in Spain)14 between 1997 and 2008, showed an annual hospitalisation rate of 2.33 per 100,000 inhabitants with an associated direct annual cost of more than 5 million euro.14
For survivors affected by permanent sequelae, the latter put a considerable burden on them and their families from both an economic and a quality of life perspective that must be taken into account when deciding whether or not the vaccine should be included in the routine schedule. A study conducted in Spain showed that the short- and long-term costs associated with surviving patients that have sequelae from IMD constitute a considerable economic burden. The total cost for severe long-term sequelae associated with septicaemia amounts to 2,515,554 euro, and the cost for severe long-term sequelae associated with meningitis to 3,219,653 euro.15
Vaccine against serogroup B meningococcal diseaseThe Bexsero®4CMenB vaccine, based on four components (NadA, fHbp, NHBA and OMVnz), was designed through an innovative approach termed “reverse vaccinology”.
It is administered intramuscularly as a 0.5mL dose. Table 2 shows its composition.16 It must be kept refrigerated between +2°C and +8°C, and it cannot be frozen.
Meningococcal group B vaccine (4CMenB, Bexsero®): composition according to summary of product characteristics (last accessed: August 22, 2014).16
| – 50μg of recombinant Neisseria meningitidis group B NHBA fusion protein– 50μg of recombinant Neisseria meningitidis group B NadA protein– 50μg of recombinant Neisseria meningitidis group B fHbp protein– 25μg of outer membrane vesicles (OMVs) from Neisseria meningitidis group B strain NZ98/254, measured as amount of total protein containing the PorA P1.4.Components are adsorbed on 1.5mg of aluminium hydroxide; it also contains 3.25mg of ClNa |
According to the summary of product characteristics of the Bexsero® vaccine, currently approved in Europe and by extension in Spain, it is indicated for active immunisation against IMD caused by serogroup B N. meningitidis starting at 2 months of age.3 Thus, the vaccine is approved for administration in both healthy and at-risk individuals older than 2 months.16
Immunogenicity, efficacy and effectivenessThe experience of 10 clinical trials that assessed the immunogenicity of this vaccine comprehended approximately 5800 individuals, of whom 4000 were children aged 2–24 months, 84 children aged 40–43 months, and 1738 adolescents or adults aged 11–55 years.8 These studies demonstrated that the vaccine is immunogenic and safe in all of these age groups, and that it induces immunological memory.
It is very difficult to demonstrate efficacy in clinical trials and population effectiveness in the prevention of a disease as rare as this one. Only the introduction of the vaccine in routine immunisation schedules and subsequent epidemiologic surveillance could prove its effectiveness in vaccinated and unvaccinated populations, similar to what occurred with the vaccines against group C meningococcus or Haemophilus influenzae type b. It is unlikely that it will be any different with the MenB. The effectiveness data from the United Kingdom will probably be the earliest evidence on this subject, as this country has already introduced the vaccine into its routine immunisation schedule.17 Furthermore, more data on the duration of immunogenicity will become available in upcoming years that may influence future changes in immunisation guidelines, as occurred with the meningococcal C vaccine.
Coverage and potential impact on disease in SpainThe Meningococcal Antigen Typing System (MATS) is a method developed to assess the potential coverage of the 4CMenB vaccine against circulating strains of MenB, although it could be used for other vaccines. This method is used to find out the percentage of meningococcal strains that could be killed by the antibodies contained in the vaccine.
The MATS involves a vaccine antigen-specific enzyme-linked immunosorbent assay (ELISA) that detects qualitative and quantitative differences in the expression of these antigens. It measures both the immunologic cross-reactivity and quantity of the expressed NHBA, NadA and fHbp antigens. It also includes PorA genotyping information to assess the potential coverage associated with this antigen. The results obtained with the ELISA were correlated with the killing of strains by SBA.
In a study conducted with MenB strains isolated in several European countries (Germany, France, England and Wales, Italy, Norway, Spain and Portugal), MATS was used to predict that the 4CMenB vaccine would cover 73–87% of the strains.18 The data obtained in Spain, which included 300 strains, predicted a coverage of 69% (95% CI, 48.0–85.3%),18,19 slightly below the coverage estimated for the other European countries under study. The estimated coverage in Spain was similar to that of the United Kingdom (73%; 95% CI, 58%–87%), for which 535 strains were analysed.18
Based on the five epidemic seasons from 2007–2008 to 2011–2012, which resulted in 914 cases in children younger than 5 years and a total of 6933 disability-adjusted life years (DALYs), and considering the potential effectiveness percentage of 69% predicted by the MATS data, the vaccine could prevent up to 631 cases in the next five seasons and a total of 4784 DALYs in Spain for an assumed vaccine coverage of 100%.4
SafetyThe safety of this vaccine has been evaluated in nine clinical trials that included a total of 4800 infants younger than 12 months, 1600 toddlers aged 12–24 months, 84 children aged 40–43 months, and 1738 adolescents and adults aged 11–55 years. The most frequent local and systemic adverse reactions observed in children younger than 24 months were pain and redness at the injection site, fever and irritability. Clinical trials in infants showed that the fever developed more frequently when the 4CMenB vaccine was coadministered with routine vaccines (61%) than when given alone (38%) or compared to routine vaccines administered alone (33%).16 The fever is usually mild, with onset in the first 6h and rarely lasting past 36–48h. A fever higher than 38.5°C was observed in 37% of children older than 12 months and in only 2–5% of adolescents.
When it comes to the use of antipyretics, one study showed that the prophylactic use of paracetamol reduced the likelihood of developing a fever and other local and systemic adverse effects without diminishing the immune response to the administered vaccines.20
Coadministration with other vaccinesThe published literature shows that Bexsero® is compatible with routine vaccines, except for the meningococcal C vaccine,16,21 for which there is a study underway the results of which will be available in 2015. It is also authorised for coadministration with the pneumococcal 13-valent conjugate vaccine (Prevenar 13®), as well as the rotavirus22 and the varicella16 vaccines.
Current situation of the vaccine in Spain and in the rest of EuropeThe 4-component (NadA, fHbp, NHBA and OMVnz) 4CMenC vaccine, Bexsero®, was authorised in the EU by a centralised procedure in January 14, 2013 on the back of the positive reports of the official European evaluating agency, the EMA.3
After the centralised approval of the vaccine by the EMA, the regulatory agencies of each country had to determine the conditions for its commercial distribution.
Situation in Spain (as of August 2014)The AEMPS stated its position in regards to the vaccine on April 5, 2013: “Based on the lack of data on the clinical efficacy of this vaccine, combined with the present reduced incidence of serogroup B meningococcal disease, and awaiting post-authorisation data on its effectiveness, and in the absence, at the moment, of official recommendations for its use from the Directorate of Public Health, it is recommended that the Bexsero® vaccine be restricted to hospital use until the Directorate of Public Health, in the context of the Consejo Interterritorial del Sistema Nacional de Salud (Interterritorial Council of the National Health Service), establishes the objectives or policy to follow in the use of this vaccine”.5
Similarly, on June 20, 2013 the working group on MenB of the Ponencia de Programas y Registro de Vacunaciones del Ministerio de Sanidad, Servicios Sociales e Igualdad (Vaccination Programmes and Registry Communications Group of the Ministry of Health, Social Services and Equality) determined that “taking into account the available information and the epidemiological situation, the inclusion of this vaccine in the routine immunisation schedule is not considered justified at the present time. It is recommended that evaluation of this vaccine resumes once key information becomes available, especially as it pertains to clinical protection in geographical regions or countries in our area, and that a laboratory surveillance system is set up to determine the effectiveness of vaccination and the evolution of the N. meningitidis population. The healthcare authorities will evaluate the use of the vaccine in the event of outbreaks and isolated cases of serogroup B meningococcal disease”.4
Unfortunately, these arrangements make the situation of the Bexsero® vaccine in Spain totally different from that in the other European countries. In Spain, the vaccine has been available officially since August 13, 2014,23 but only in private and public hospital pharmacies. Which is to say, it cannot be acquired in community pharmacies.
Based on the directives of the Ministry of Health, at present the only official recipients of this vaccine are individuals with risk factors for IMD (individuals with a complement deficiency or undergoing treatment with eculizumab, with asplenia or severe splenic dysfunction, or with more than one prior episode of IMD, or laboratory staff that manipulate samples that may contain meningococci) or the vaccine can be used in the event of outbreaks.4,5 In the first case, recipients represent a very small percentage of the individuals that have this disease, so the expected impact of vaccination would be very small. Paradoxically, there is a lack of immunogenicity studies precisely in patients that are at risk for IMD, while there are studies in healthy individuals (younger than 50 years) for whom the vaccine has not been authorised in Spain.
Situation in Europe (as of August 2014)The situation of the 4CMenB in the rest of the European Union (EU) countries is completely different, as all of them, unlike Spain, have authorised it for its unrestricted distribution in community and hospital pharmacies.
In the United Kingdom the vaccine has been included in the routine immunisation schedule. In March 2014 this country approved its inclusion in the immunisation programme with a 2+1 schedule (at 2, 4 and 12 months) after reviewing all the available documentation and analysing its cost-effectiveness.24
Poland and Austria are well ahead in the process of adding the vaccine to their immunisation schedules, and other countries and regions, such as the Czech Republic, Saxony (in Germany) or Apulia, Basilicata and Tuscany (in Italy) have already included it in theirs.
Other countries, such as Germany and France, are currently analysing all the available information.25,26
Situation outside of Europe (August 2014)The possibility of routine vaccination of children younger than 24 months and adolescents 15–19 years of age as well as at-risk groups is being considered in Australia.27 In the United States the vaccine is yet to be approved by the Food an Drug Administration, but it was used on an emergency basis in over 14,000 students due to independent outbreaks of serogroup B meningococcal disease in two universities (Princeton and UC Santa Barbara).28,29 In Canada, which had a mean incidence rate of 0.57 cases per 100,000 inhabitants per year for the 2007–2011 period, it was decided to implement routine vaccination of the population aged 2 months to 20 years in Quebec.30
Approved posology in EuropeThe vaccine is indicated for immunisation against IMD caused by serogroup B N. meningitidis starting at 2 months of age.3Table 3 shows the posology currently recommended in the summary of product characteristics approved by the EMA.16
Recommended posology for the vaccine against group B meningococcus (4CMenB, Bexsero®), according to summary of product characteristics.16
| Age group | Primary immunisation, number of doses | Intervals between primary doses | Booster doses |
|---|---|---|---|
| Infants, 2 months to 5 months | 3a | Not less than 1 month | Yes, one dose between 12 and 15 months |
| Unvaccinated infants, 6 months to 11 months | 2 | Not less than 2 months | Yes, one dose in the second year of life with an interval of at least 2 months between the primary series and the booster doseb |
| Unvaccinated children, 12 months to 23 months | 2 | Not less than 2 months | Yes, one dose with an interval of 12 months to 23 months between the primary series and booster doseb |
| Children, 2 years to 10 years | 2 | Not less than 2 months | Need not established |
| Adolescents (from 11 years of age) and adults | 2 | Not less than 1 month | Need not established |
Final considerations and recommendations of the Comité Asesor de Vacunas de la Asociación Española de Pediatría on the four-component meningococcal serogroup B vaccine (4CMenB)
Table 4 summarises the main data on group B meningococcal disease and its prevention by means of vaccination.
Summary of published data on invasive meningococcal disease by serogroup B and its prevention by means of vaccination with 4CMenB (Bexsero®).
| – The incidence of IMD by serogroup B in Spain is 0.7 cases per 100,000 inhabitants per year, one of the highest in Europe. It is much more common and has a poorer prognosis in children younger than 2 or 3 years, followed by adolescents, although it may occur at any age.– The mortality of this disease is approximately 10%. The probability of long-term sequelae, mostly neurological sequelae and amputations, is 20–30%– The 4CMenB is immunogenic and safe in infants, children, adolescents and adults and induces immunological memory.– The systemic reactogenicity of the 4CMenB vaccine (fever) is greater than that of other routine vaccines, especially if when coadministered with the latter (although it is similar to that of routine vaccines when given alone.) The fever follows a predictable and self-limiting pattern (onset at 6h, peak on day 2, resolution by day 3), is of little clinical significance, and may be prevented with the administration of paracetamol prior to vaccination.– The 4CMenB vaccine can be given with any of the vaccines included in the routine immunisation schedules of all autonomous communities and with vaccines not included in them, such as the pneumococcal, rotavirus, and varicella vaccines. The exception is the meningococcal C vaccines (pending completion of a current study).– The potential coverage of the 4CMenB vaccine against strains circulating in Spain is estimated at approximately 70%, similar to other European countries.– At present, the 4CMenB vaccine is the only available strategy to prevent group B meningococcal disease, and it has been approved for its use in individuals from 2 months of age.– The 4CMenB vaccine is starting to be administered as part of the official immunisation schedule in some parts of the world, such as the United Kingdom, Saxony (Germany), Puglia, Basilicata and Tuscany (Italy), the Czech Republic, Quebec (Canada) and Australia. It is very likely that other countries will follow suit in upcoming months.– Although the EMA has approved its free distribution throughout the EU, in Spain it is only authorised for hospital use, so it is not available in community pharmacies. |
Table 5 presents the considerations and recommendations of the CAV-AEP on this vaccine at the time this paper was being written (August 2014). The committee believes that this vaccine has the profile of a routine vaccine that should be included in the schedules of every autonomous community in Spain, and that the vaccine should be available for its unrestricted distribution to any individual older than 2 months of age.
Considerations and recommendations of the Comité Asesor de Vacunas de la AEP on the vaccine against group B meningococcal disease (4CMenB, Bexsero®) (August 2014).
| 1. The approval of the new vaccine against serogroup B meningococcal disease by the EMA opens up new horizons in the prevention of meningococcal disease by this serogroup, which is the most prevalent in all of Europe, including Spain.2. The CAV-AEP considers that this vaccine has the profile of a routine vaccine to be included in the calendars of all the autonomous communities in Spain. The prevention of deaths and permanent sequelae caused by this severe disease justifies recommending this vaccine, regardless of cost-effectiveness analyses.3. If it were to be included in the routine immunisation schedules, the most appropriate way to incorporate it to the schedules currently in force should be investigated, respecting the specifications and technical indications for each of the vaccines that are already included.4. If it were not included in the official routine schedules, the vaccine should be made available for its use by healthcare professionals when they consider it appropriate. The vaccine has been approved by the EMA for its use in any individual starting at 2 months of age.5. The posology recommended by the CAV-AEP is the one specified in the summary of product characteristics (Table 3). A 3+1 schedule is recommended in children starting vaccination within 5 months from birth. The inclusion of the vaccine in the routine immunisation schedule could allow the use of a 2+1 schedule, as decided by the authorities of the United Kingdom.6. The frequent development of fever as an adverse reaction to the administration of this vaccine should not be a barrier to its recommendation. Although there are data that support the prophylactic administration of antipyretics, this committee continues to recommend limiting the use of antipyretics for treating symptoms, whenever it is appropriate.7. The committee urges the public health authorities to reconsider their decision to restrict this vaccine to hospital use, and request that it the vaccine is made available in community pharmacies to anyone wishing to acquire it after receiving a well-informed recommendation and prescription from their paediatrician.8. The committee recommends the exhaustive monitoring and evaluation of all information on the efficacy, effectiveness and compatibility with other vaccines in the schedule, which will start to become available in upcoming months from those countries or regions that have decided to include the vaccine in their routine immunisation schedules, such as the United Kingdom and some regions in Germany and Italy, in order to support recommendations that most benefit all children. |
The conflicts of interest of the authors in the past five years are the following:
- -
D. Moreno-Pérez has collaborated in educational activities funded by Glaxo Smith Kline, Novartis, Pfizer and Sanofi Pasteur MSD, participated as a researcher in clinical trials for Novartis, and as an Advisory Board member for Astra-Zeneca, Novartis and Pfizer.
- -
F.J. Álvarez García has collaborated in educational activities funded by Glaxo Smith Kline, Novartis, Pfizer and Sanofi Pasteur MSD and as an Advisory Board member for Novartis.
- -
J. Arístegui Fernández has collaborated in educational activities and as a researcher in clinical trials funded by Glaxo Smith Kline, Pfizer and Sanofi Pasteur MSD, and as an Advisory Board member for Novartis.
- -
M.J. Cilleruelo Ortega has collaborated in educational activities funded by Glaxo Smith Kline, Novartis, Pfizer and Sanofi Pasteur MSD, as a researcher in clinical trials for Pfizer and as an Advisory Board member for Novartis.
- -
J.M. Corretger Rauet has collaborated in educational activities funded by Glaxo Smith Kline, Sanofi Pasteur MSD and Novartis.
- -
N. García Sánchez has collaborated in educational activities funded by Sanofi Pasteur MSD and attended educational events funded by Novartis and Pfizer.
- -
A. Hernández Merino has received grants to attend educational events in Spain.
- -
T. Hernández-Sampelayo Matos has collaborated in educational activities funded by Glaxo Smith Kline, Pfizer and Sanofi Pasteur MSD and as a researcher in clinical trials funded by Glaxo Smith Kline and Pfizer.
- -
M. Merino Moína has collaborated in educational activities funded by Glaxo Smith Kline, Pfizer and Sanofi Pasteur MSD, as a researcher in clinical trials for Glaxo Smith Kline, Pfizer and Sanofi Pasteur MSD and as an Advisory Board member for Novartis.
- -
L. Ortigosa del Castillo has collaborated in educational activities funded by Glaxo Smith Kline, Novartis, Pfizer and Sanofi Pasteur MSD and as a researcher in clinical trials for GlaxoSmithKline.
- -
J. Ruiz-Contreras has collaborated in educational activities funded by Glaxo Smith Kline, Pfizer and Sanofi Pasteur MSD and as a researcher in clinical trials for Glaxo Smith Kline and Pfizer.
Please cite this article as: Moreno-Pérez D, Álvarez García FJ, Arístegui Fernández J, Cilleruelo Ortega MJ, Corretger Rauet JM, García Sánchez N, et al. Vacunación frente al meningococo B. Posicionamiento del Comité Asesor de Vacunas de la Asociación Española de Pediatría. An Pediatr (Barc). 2015;82:198.e1–198.e9.