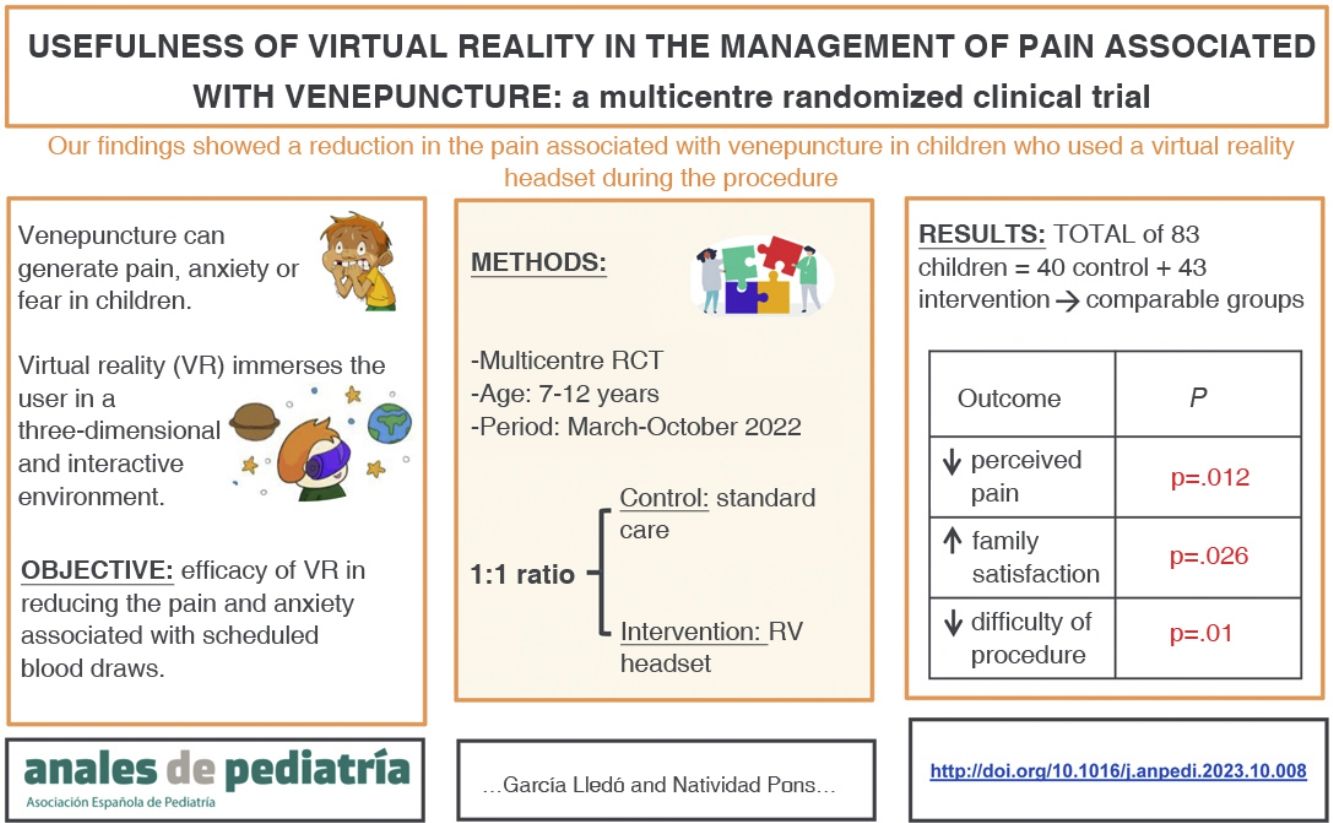
To assess the efficacy of virtual reality (VR) in reducing pain and anxiety associated with scheduled blood draws.
Material and methodsWe conducted multicentre randomized clinical trial in both primary care and hospital settings. The study included children aged 7–12 years undergoing blood extraction procedures between March and October 2022. The intervention group used headsets, and the control group received usual care. Pain was assessed using the visual analogue scale and anxiety using the Groningen Distress Scale. The anxiety of the nursing staff and family satisfaction were assessed with numerical scales ranging from 1 to 10.
ResultsThe study included 83 patients: 40 in the control group and 43 in the VR group. The median age was 10 years (range, 7−12 years). In the VR group, 83.7% of the children reported mild pain, compared to 57.5% in the control group (P = .012). Also, 93% of children in the VR group showed calm or mild anxiety (score, 1−2), compared to 72% of the control group, a difference that was not statistically significant (P = .08). Family satisfaction was higher in the RV group (score ≥ 9/10: 93% of RV group vs 72.5% of control group; P = .026). The nursing staff anxiety score was less than 5 in more than 90% of cases, with no differences between groups (P = .13).
ConclusionThe use of VR during venepuncture decreases the pain perceived by children and increases the satisfaction of their families.
Comprobar la eficacia de la realidad virtual (RV) en la disminución del dolor y ansiedad asociados a extracciones sanguíneas programadas.
Material y MétodosEnsayo clínico aleatorizado multicéntrico realizado en atención primaria y hospitalaria. Se incluyeron los pacientes de 7 a 12 años que acudieron entre marzo y octubre de 2022 a realizarse una extracción sanguínea programada.
En el grupo intervención se utilizaron gafas de RV y en el grupo control cuidados habituales. Se evaluó el dolor mediante la Escala Visual Analógica y la ansiedad mediante la Groningen Distress Scale. Se evaluó la ansiedad de enfermería y la satisfacción de los familiares, mediante escala numérica del 1 al 10.
ResultadosSe incluyeron 83 pacientes: 40 en el grupo control y 43 en el grupo RV. La mediana de edad fue de 10 años (rango de 7 a 12). El 83,7% de los niños en el grupo RV refirió dolor leve, frente al 57,5% del grupo control (p = 0,012). El 93% del grupo RV mostró calma o ansiedad leve (puntuación 1−2), frente al 72% del grupo control, aunque sin diferencias estadísticamente significativas (p = 0,08).
La satisfacción de los familiares fue mayor en el grupo RV (≥9/10: 93% de RV vs 72,5% de controles, p = 0,026). La ansiedad de enfermería fue < 5 en más del 90% de los casos, sin diferencias entre grupos (p = 0,13).
ConclusionesEl uso de RV durante la venopunción disminuye el dolor percibido por los niños y aumenta la satisfacción de sus familiares.
Certain procedures, such as routine blood draws, can cause pain, anxiety or fear in children. Pharmacological and non-pharmacological sedation and analgesia techniques are available to manage procedural pain and anxiety.
One of the most commonly used pharmacological methods to manage procedural pain is the administration of topical anaesthetics such as lidocaine/prilocaine. For procedural anxiety, drugs like nitrous oxide or intranasal midazolam may be useful.1 For procedures that cause severe pain, drugs like morphine, fentanyl or ketamine can be administered intravenously.
Non-pharmacological methods can be combined with pharmacological methods. They can be classified into2: support methods (presence of family members), cognitive methods (active and passive distraction), behavioural methods (deep breathing) and physical methods (local cold therapy).
A literature review that evaluated the evidence published between 2005 and 20153 found that certain toys or games may be effective in reducing the pain and anxiety associated with venepuncture. Some of them were musical tables, videogames, virtual reality (VR) goggles and kaleidoscopes.
Virtual reality is an active distraction method. It is defined as an immersive, interactive and three-dimensional computer-generated environment.4 Immersion in VR helps to reduce the awareness of painful stimuli, which offers an advantage compared to other distraction methods.
Different devices are available to use VR.4 Some headsets have an integrated screen and headphones and others require the attachment of a mobile phone. The latter tend to be more affordable and may be used since 4 years old. Headsets with integrated screens and headphones are not appropriate for younger children, since the interpupillary distance setting cannot be adjusted.
Numerous studies have assessed the use of VR for management of procedural pain in the paediatric population.5–9 Most have been conducted in inpatient or emergency care settings in patients who underwent catheter placement or blood tests.5–7 Few studies have assessed the use of VR in routine blood draws, and most of them have been conducted in hospital settings.8,9
The aim of our study was to test the efficacy of VR in reducing pain and anxiety associated with routine blood sample collection in paediatric patients in both the hospital and primary care settings.
Material and methodsStudy designWe conducted a multicentre open-label randomized clinical trial with participation of 5 primary care centres and the 2 regional hospitals located in the same health area of the Valencian Community, Spain. The study was carried out in adherence to the CONSORT guidelines.10 Patients were allocated in a 1:1 ratio to the intervention group (use of VR during blood draw) or the control group (standard care).
Population, sample and randomizationWe calculated the sample size out of the total population aged 7–12 years who had undergone routine blood draws in our health area in 2019 required for a power of 90%. The calculated minimum was of 80 patients.
Eligible candidates were patients who visited participating centres to undergo blood tests during the recruitment period (March to October 2022).
We recruited children aged 7–12 years who assented and whose parents or legal guardians consented to participation in the study by signing the informed consent form. The minimum age was set at 7 years because visual development is complete, and the maximum at 12 years to avoid an overly broad age range, so that the same VR video could be used for the entire sample.
We excluded patients with psychomotor or neurodevelopmental delay, in whom assessment of anxiety would be complicated. Patients with visual or hearing impairments were also excluded, as we were unable to adapt the RV headset to their particular conditions.
To carry out the study, we randomised patients by means of the Research Randomizer software tool (https://www.randomizer.org/). The tool generated 7 sequences (one per participating centre) that randomly assigned the numbers 1 (intervention) and 2 (control) to 30 specific positions. After obtaining the 7 sequences, the principal investigator prepared 30 closed envelopes containing the words “intervention” or “control”, following the order established by the randomly generated sequences. The allocation envelopes were marked successively with numbers 1 through 30 so that the nurses, after recruiting patients, would open the corresponding envelope and know the group the patient had been allocated to. This way, nurses did not know which group the patient was going to be allocated to until they had been recruited.
The study protocol consisted of:
- 1
Informing the patient.
- 2
Obtaining signed informed consent.
- 3
Opening of the envelop specifying the group allocation.
- 4
Blood draw conducted by the standard procedure or with VR depending on group allocation.
At the time of enrollment, each patient was assigned a code for data pseudonymization.
Study protocolIn the intervention group, patients wore an Oculus Quest 2 VR headset (Meta, California, USA) that played a video from the moment the supplies started to be prepared to the moment pressure was applied to the puncture site. The video was a short film titled “Henry”. It is a cartoon aimed at young children in which the main character is a hedgehog that celebrates his birthday with animal-shaped balloons that come alive.
In the control group, venepuncture was carried out per the standard protocol (distraction method with nurses talking to the patient).
All the blood draws were performed by the same nurses. Before recruitment, all the nurses that would collaborate in the study attended a meeting. One or two nurses were included per participating centre; they were nurses usually tasked with performing routine blood draws in their respective facilities and the only ones who performed blood draws included in the study. In the meeting, they were trained on the use of the RV headset and informed of the study protocol. They also received all the material required for the study (headset and documentation).
Study variablesIndependent variables:
- -
Age
- -
Sex
- -
History of recent venepuncture (<6 months)
- -
Presence of family members
Outcome variables:
- none-
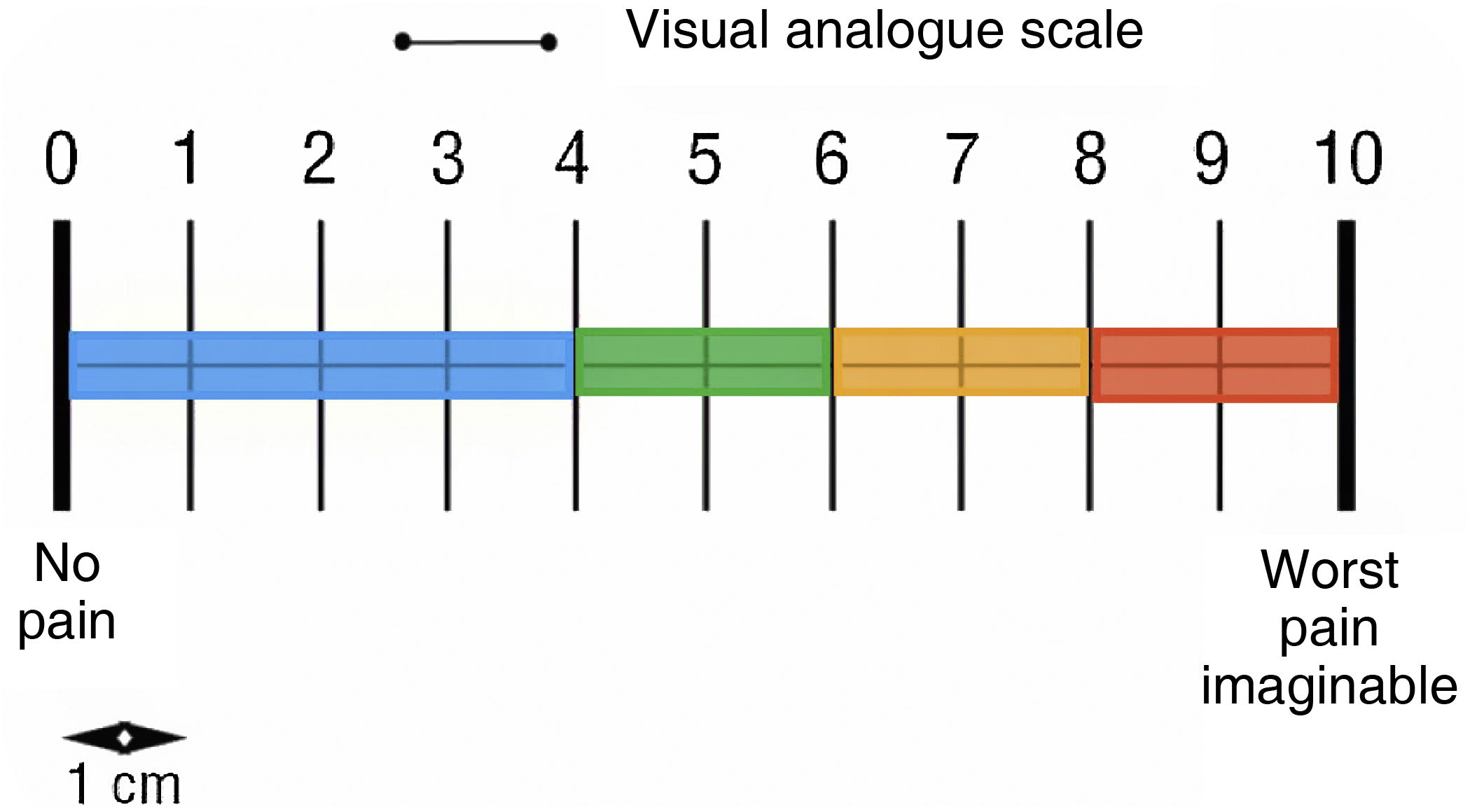
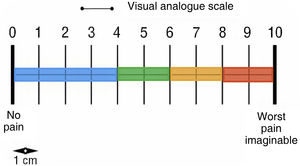
Pain (visual analogue scale) (Fig. 1).
- none-
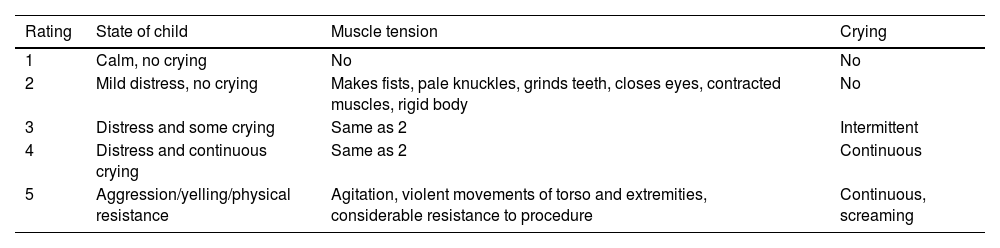
Patient anxiety (Groningen Distress Scale) (Table 1).
Table 1.Groningen distress scale.
Rating State of child Muscle tension Crying 1 Calm, no crying No No 2 Mild distress, no crying Makes fists, pale knuckles, grinds teeth, closes eyes, contracted muscles, rigid body No 3 Distress and some crying Same as 2 Intermittent 4 Distress and continuous crying Same as 2 Continuous 5 Aggression/yelling/physical resistance Agitation, violent movements of torso and extremities, considerable resistance to procedure Continuous, screaming - none-
Number of attempts required for extraction.
- none-
Time required for the technique (minutes), per attempt, from application of tourniquet to compression of puncture site.
- none-
Degree of difficulty of the draw as perceived by the nurse (rated as easy, normal or difficult).
- none-
Family satisfaction (numerical scale from 1 to 10, with 1 representing “not at all satisfied” and 10 “very satisfied”).
- none-
Anxiety experienced by the nurse who carried out the procedure (numerical scale from 1 to 10, with 1 representing “totally calm” and 10 “very anxious or nervous”).
The study was approved by the competent ethics committee (CEIm 02/2022, project no 37/2021). All participants and their family members were informed and signed the consent form to participate in the study. The study was registered in ClinicalTrials.gov with NCT number NCT05902585.
Statistical analysisThe collected data were entered in a database created for the purpose with the software SPSS version 26 (IBM Corp, Armonk, NY, USA), safeguarding the confidentiality of the patients.
We carried out a descriptive analysis of the data. Quantitative variables were expressed as mean and standard deviation if the data followed a normal distribution, or otherwise as median and range. Qualitative variables were expressed as absolute frequency and percentage distributions.
To assess the association between two variables, we compared frequencies by means of the Fisher exact test in the case of comparing 2 dichotomous variables and the χ2 test in the case of comparing variables with more than 2 categories. Since a nonparametric test was required, we used the Mann–Whitney U test to assess if there were differences in individual variables between groups, and we analysed the association between two variables with the Spearman correlation coefficient.
To estimate the number needed to treat (NNT), we calculated the reduction in absolute risk for mild pain and anxiety between the intervention and control groups. The level of statistical significance was established at 0.05 for all statistical tests.
ResultsCharacteristics of the sampleThe sample included 83 patients with a mean age of 9.7 years (SD, 1.7), of who 44.6% were female.
After random allocation, 40 patients were included in the control group (standard care) and 43 in the intervention group (VR). There were no losses to follow-up.
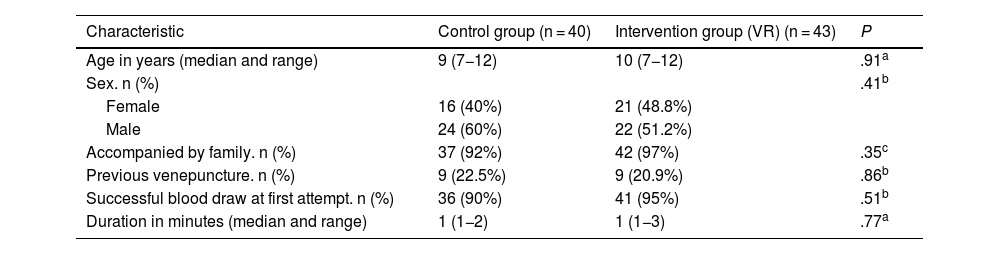
Both groups were comparable, as there were no statistically significant differences in their characteristics (Table 2); 21.7% of participants had undergone venepuncture in the past 6 months. Ninety-five percent of the children were accompanied by a relative during the procedure.
Characteristics of each group.
| Characteristic | Control group (n = 40) | Intervention group (VR) (n = 43) | P |
|---|---|---|---|
| Age in years (median and range) | 9 (7−12) | 10 (7−12) | .91a |
| Sex. n (%) | .41b | ||
| Female | 16 (40%) | 21 (48.8%) | |
| Male | 24 (60%) | 22 (51.2%) | |
| Accompanied by family. n (%) | 37 (92%) | 42 (97%) | .35c |
| Previous venepuncture. n (%) | 9 (22.5%) | 9 (20.9%) | .86b |
| Successful blood draw at first attempt. n (%) | 36 (90%) | 41 (95%) | .51b |
| Duration in minutes (median and range) | 1 (1−2) | 1 (1−3) | .77a |
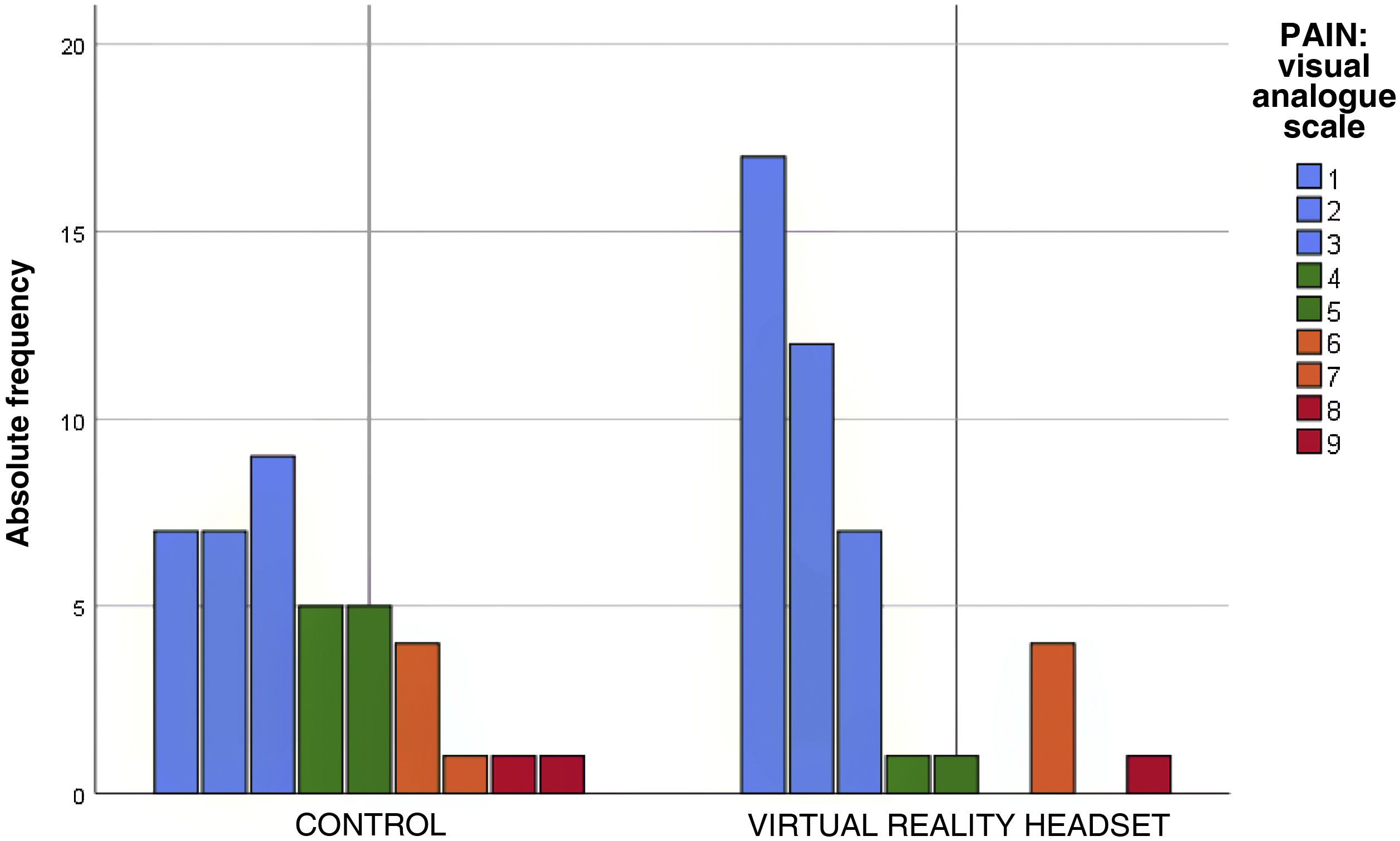
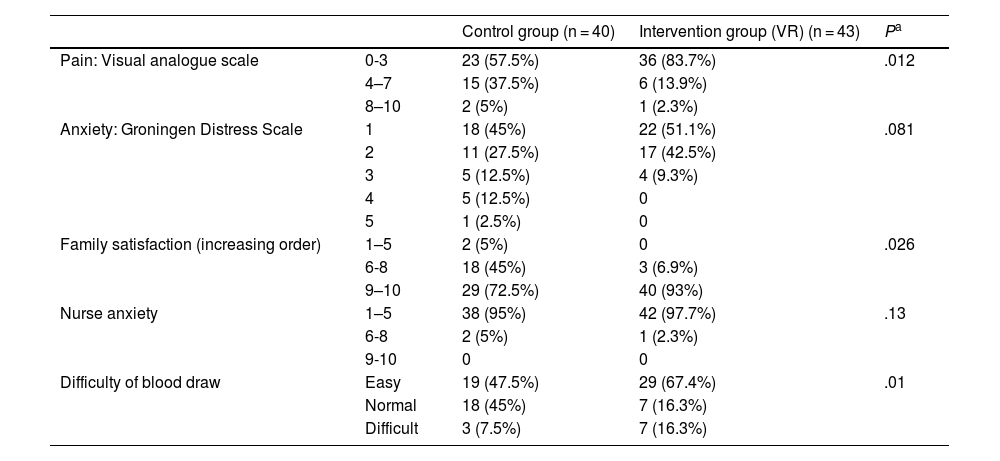
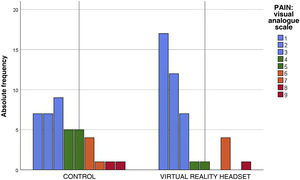
The perceived pain, assessed with the visual analogue scale, was milder in the VR group (Fig. 2), a difference that was statistically significant (P = .012) (Table 3). In the VR group, 83.7% of children reported absent or mild pain compared to 57.5% of controls. This corresponds to a 26% reduction in the frequency of pain in children, and therefore, the NNT for one child to report mild pain is of 4 children.
Anxiety and pain in scheduled blood draws.
| Control group (n = 40) | Intervention group (VR) (n = 43) | Pa | ||
|---|---|---|---|---|
| Pain: Visual analogue scale | 0-3 | 23 (57.5%) | 36 (83.7%) | .012 |
| 4–7 | 15 (37.5%) | 6 (13.9%) | ||
| 8–10 | 2 (5%) | 1 (2.3%) | ||
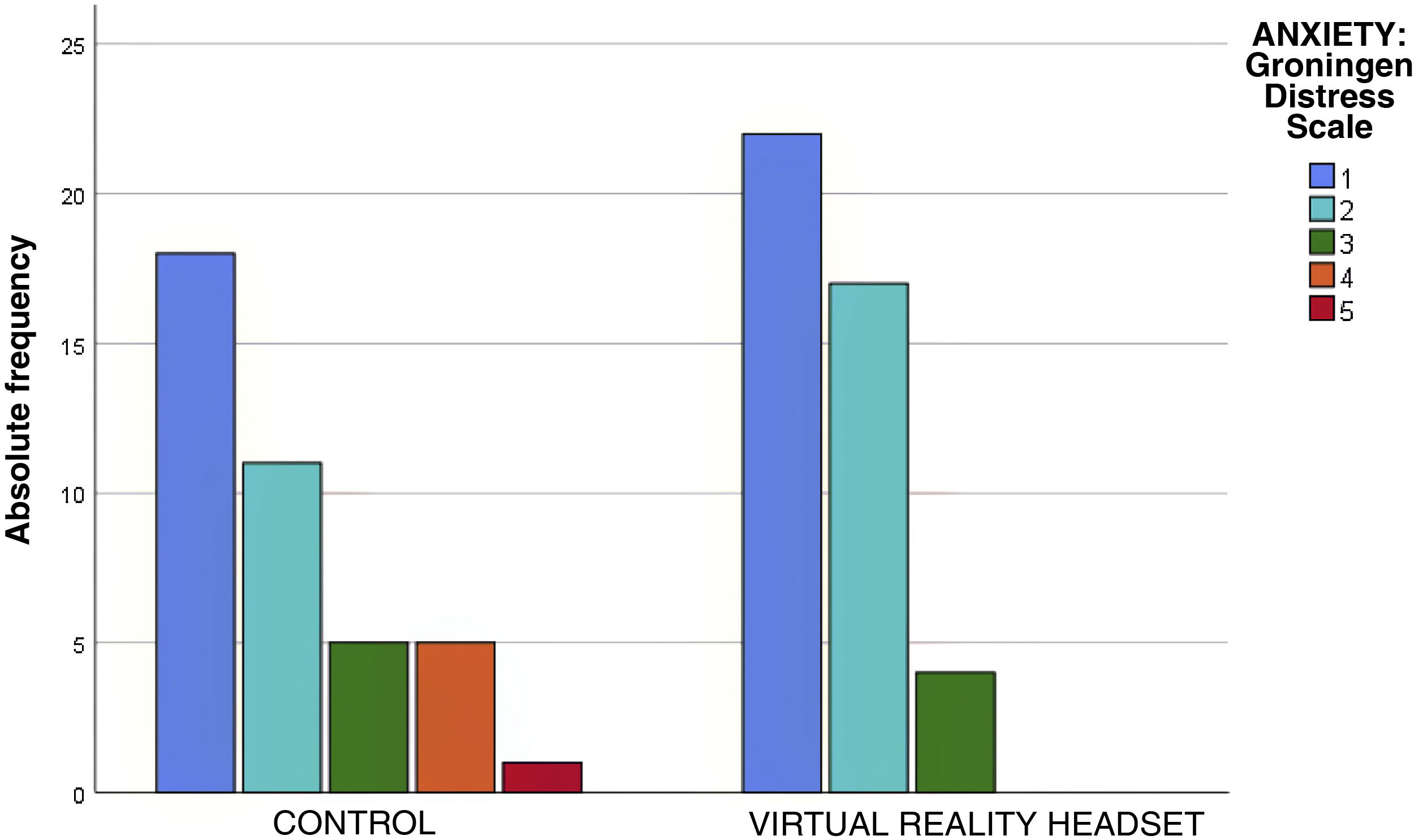
| Anxiety: Groningen Distress Scale | 1 | 18 (45%) | 22 (51.1%) | .081 |
| 2 | 11 (27.5%) | 17 (42.5%) | ||
| 3 | 5 (12.5%) | 4 (9.3%) | ||
| 4 | 5 (12.5%) | 0 | ||
| 5 | 1 (2.5%) | 0 | ||
| Family satisfaction (increasing order) | 1–5 | 2 (5%) | 0 | .026 |
| 6-8 | 18 (45%) | 3 (6.9%) | ||
| 9–10 | 29 (72.5%) | 40 (93%) | ||
| Nurse anxiety | 1–5 | 38 (95%) | 42 (97.7%) | .13 |
| 6-8 | 2 (5%) | 1 (2.3%) | ||
| 9-10 | 0 | 0 | ||
| Difficulty of blood draw | Easy | 19 (47.5%) | 29 (67.4%) | .01 |
| Normal | 18 (45%) | 7 (16.3%) | ||
| Difficult | 3 (7.5%) | 7 (16.3%) |
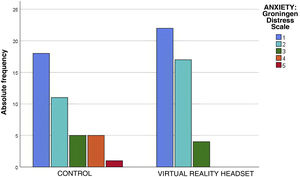
The assessment of anxiety by means of the Groningen Distress Scale found calm (rating of 1) or mild anxiety (rating of 2) in 81% of the children, without statistically significant differences between groups (P = .08) (Table 3). However, in the control group there were 6 patients with scores greater than 3 on the Groningen Distress Scale, while there were no patients with this score in the intervention group (Fig. 3). Of the children who wore the headsets, 93% reported no anxiety or mild anxiety, compared to 72% of controls. Therefore, the NNT to reduce anxiety in one child with VR is of 6 children.
In 93% of cases, the blood draw was successful on the first attempt, only five children required a second attempt and one more than 2 attempts. The mean time elapsed from the application of the tourniquet to compression after completion of the blood draw was of 1 min and 40 s, with no differences between groups (Table 2).
Based on the nurse reports, 58% of the blood draw procedures were easy, 30% had the average level of difficulty and 12% were difficult.
Family members reported a satisfaction greater than or equal to 9 in 93% of cases in the VR group compared to 72.5% in the control group (P = .026). None of the patients experienced adverse effects from the use of VR, although some participants reported that the headset felt too heavy.
Nurses rated their level of anxiety below 5 points (on a scale from 1 to 10) for more than 90% of blood draws in both groups (P = .13) (Table 3). However, nurses reported difficulty performing the procedure less frequently with the use of VR headsets (P = .01).
In respect of the care setting, 77% of blood draws were performed in primary care and 23% in hospital. We did not find differences in the pain ratings based on the setting where the procedure was performed (P = .13). Children also did not experience greater anxiety at the hospital (P = .37). There were also no differences in the anxiety or the difficulty reported by the nurse performing the procedure (P = .15 and P = .13, respectively). Family satisfaction was high in both care settings (P = .10) in both the intervention and control groups.
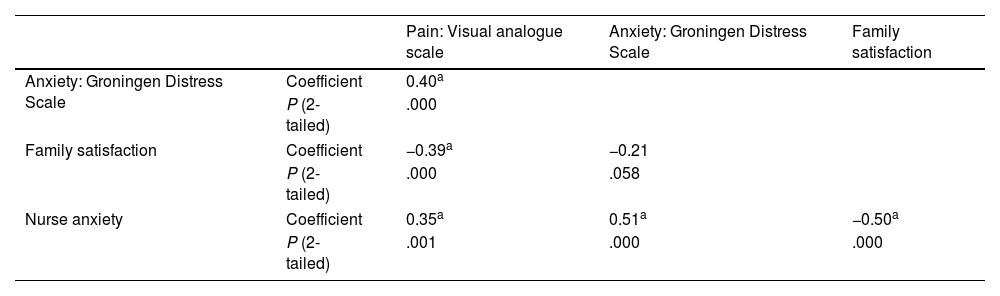
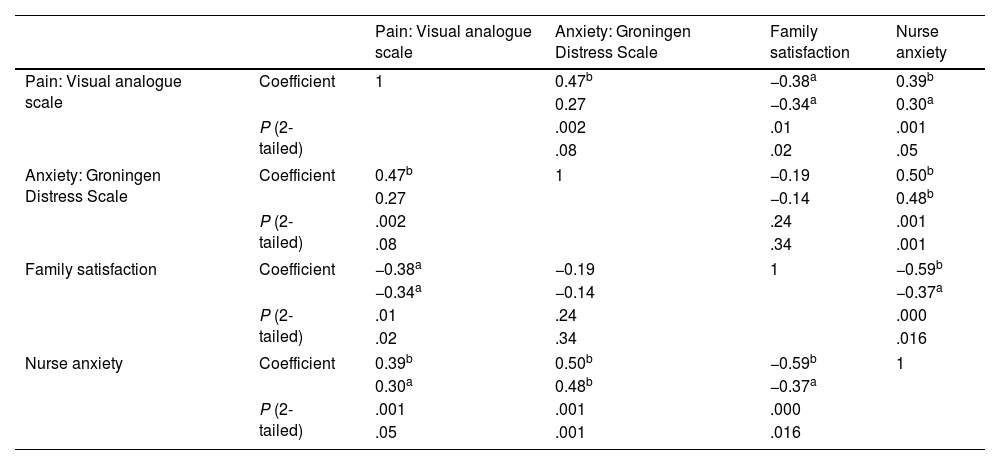
In the overall sample, we found a significant correlation between the pain and the anxiety experienced by the child (rs = 0.4; P = .001 [2-tailed]), and an inverse correlation between the pain reported by the child and the satisfaction of the family (rs = −0.39; P = .001 [2-tailed]) (Table 4). Anxiety in the nurse correlated to anxiety in the child (rs = 0.5; P = .001 2-tailed) and both were inversely correlated to family satisfaction, although the association was only significant with nurse anxiety (rs = −0.5; P = .001 [2-tailed]). These associations persisted in the analysis by group, both in children who used VR headsets and in children who received standard care (Table 5), with a weaker correlation in the intervention group.
Correlation of patient anxiety and pain with nurse anxiety and family satisfaction.
Correlation analysis of patient pain and anxiety in the intervention and control groups.
| Pain: Visual analogue scale | Anxiety: Groningen Distress Scale | Family satisfaction | Nurse anxiety | ||
|---|---|---|---|---|---|
| Pain: Visual analogue scale | Coefficient | 1 | 0.47b | −0.38a | 0.39b |
| 0.27 | −0.34a | 0.30a | |||
| P (2-tailed) | .002 | .01 | .001 | ||
| .08 | .02 | .05 | |||
| Anxiety: Groningen Distress Scale | Coefficient | 0.47b | 1 | −0.19 | 0.50b |
| 0.27 | −0.14 | 0.48b | |||
| P (2-tailed) | .002 | .24 | .001 | ||
| .08 | .34 | .001 | |||
| Family satisfaction | Coefficient | −0.38a | −0.19 | 1 | −0.59b |
| −0.34a | −0.14 | −0.37a | |||
| P (2-tailed) | .01 | .24 | .000 | ||
| .02 | .34 | .016 | |||
| Nurse anxiety | Coefficient | 0.39b | 0.50b | −0.59b | 1 |
| 0.30a | 0.48b | −0.37a | |||
| P (2-tailed) | .001 | .001 | .000 | ||
| .05 | .001 | .016 | |||
In each cell, the first value corresponds to the control group and the second to the intervention group. Spearman correlation.
The history of a previous blood draw in the past 6 months was not associated with the degree of anxiety (P = .39) or pain (P = .48) in the children, nor with the difficulty of the procedure (P = .33).
DiscussionOur findings showed a statistically significant reduction in the pain associated with venepuncture, supporting the use of VR as a distraction method for the reduction of pain in children.
When it came to anxiety, although our study focused on scheduled blood draws in which the level of anxiety of patients and families is probably lower compared to emergency situations, it would have been preferable to assess anxiety before and after the procedure, and not only after.
It is interesting that only 4 children need to be treated to reduce the level of pain to mild in one child, and only 6 to reduce the level of anxiety in one child to mild or absent. This supports the efficacy of VR, although we did not analyse the extra time it takes nurses to perform the procedure with the addition of this technique.
The care setting where the procedure took place had no impact on the outcomes. A possible explanation is that all the nurses that participated in the study had more than 10 years of experience and routinely performed scheduled blood draws.
In our study, we found that pain and anxiety were correlated in patients, which may have been related to the fact that both were assessed at the end of the procedure. As expected, we found that pain and anxiety in the child were inversely correlated to family satisfaction. The level of anxiety reported by nurses increased with the level of pain and anxiety reported by the patients. This was consistent with the findings of a recent study conducted in hospitalised patients in Spain.11
The correlation persisted in the independent analyses of each group, with a lower P value in the VR group. This may be due to the reduction in pain and anxiety achieved with the use of VR.
There is previous evidence of an increase in pain associated with a previous history of painful procedures, especially those involving needles.12 However, in our study we did not find an association between the level of pain or anxiety and the history of venepuncture in the past 6 months. The absence of correlation may have been due to the small number of children that had recently undergone venepuncture.
Numerous studies have assessed the use of VR for analgesia, but they vary widely in terms of the type of patient and the type of procedure, as reflected by a meta-analysis published in 2019.13 Most studies in the literature have been conducted in the emergency care setting.5–7 In Spain, one example is a randomised clinical trial5 that assessed the pain and anxiety associated with venepuncture and peripheral catheter insertion in a group that used VR and a control group that received standard care (distraction with objects and questions about the patient’s daily life). In this study, the mean scores in pain (Wong and Baker scale) and anxiety (Groningen Distress Scale) were inferior in the VR group, although the differences were not statistically significant, probably on account of the small sample size (17 participants).
At the international level, there are studies in larger samples that allowed statistically significant results. For instance, a randomised trial conducted in 136 participants in an emergency department6 found a statistically significant reduction in pain and fear scores in the VR group. Another randomised clinical trial was conducted in 149 patients who underwent scheduled venepunctures.14 In this case, the authors found a statistically significant reduction in pain and anxiety in the VR group.
Concerning the limitations of our study, we ought to highlight the impossibility of blinding, as the nurse that managed the patient also opened the allocation envelope and fitted the VR headset for patients in the intervention group. After the blood draw, the same nurse recorded the results in the data collection form. Therefore, patients and their families as well as nurses were aware of the group to which the patient had been allocated.
A second potential limitation is selection bias, which would mainly affect the reported satisfaction of families, as the most cooperative families are the ones that accepted participation in the study. In addition, recruitment of children was difficult in some centres, as there were families that refused participation thinking that they did not have time for the entire process (information, opening of envelope and performance of the procedure). This difficulty would be less frequent in studies conducted in inpatients or in the emergency department, as families and children would not be constrained by the need to get to work or to school.
Another aspect that must be taken into account is the subjectivity intrinsic to some of the scales used in the study. The visual analogue scale and the Groningen Distress Scale have been validated in different paediatric studies.15,16 However, the scales used to assess the anxiety of nurses or family satisfaction had not been previously validated.
Last of all, the headsets used in the study were too large for some participants, so this aspect must be considered in the planning of future studies using VR.
In conclusion, the use of VR headsets during venepuncture achieved a reduction in the pain and anxiety reported by children, although the difference in anxiety was not statistically significant. Families reported greater satisfaction with the use of VR headsets, and nurses decreased difficulty in performing the procedure. Therefore, based on our experience, it is reasonable to conclude that the use of VR headsets is beneficial for the patient, the family and the health care staff.
FundingThe study was funded by the Department of Health of Xàtiva-Ontinyent.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the nurses who collaborated in the study with enthusiasm and dedication: Sandra Marchirant Alarcón, Raquel Miravalls Torres, M. Carmen Sanz Penadés, Susana Moltó Nogues, Amparo López Martorell and Amparo Soler Gandía.
Previous meeting: This study was presented as an oral communication at the 20th Meeting of Departments of Paediatrics of the Regional Hospitals of the Community of Valencia; March 2023, Vinarós, Spain.