Chronic abdominal pain (CAP) in children is a symptom that frequently leads to a visit to the paediatrician, which affects family life and occasionally requires the need to perform diagnostic studies (DS). The objective was to carry out a qualitative, quantitative, and economic analysis on the tests requested.
Material and methodsAn observational, prospective and multicentre study was conducted that included children between 4–15 years old affected by CAP. The difference between organic and functional disorders was taken into account. The following variables were collected: history, warning signs and symptoms, DS, and the cost of these.
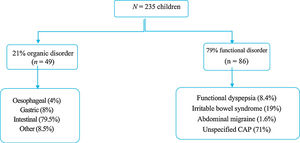
ResultsThe study included 235 children with CAP (Age; mean 9.7 ± 2.7 SD). The large majority (79%) were functional disorders and 21% organic disorders. Almost half of the patients had some warning sign or symptom, but urinary symptoms were only associated with organic disorders. The abdominal ultrasound, faecal parasites, breath test, and endoscopy were the most associated with organic disorders. There was a difference between the costs of the DS according to each centre. The total economic cost was 52,490.80 euros, with 195 euros per patient for functional disorders and 306 euros for organic disorders.
ConclusionSigns and symptoms of alarm in CAP were very frequent, but had low discriminative capacity. The abdominal ultrasound and faecal parasites are innocuous DS, and could be useful as a first level study. The endoscopy and the breath test were the most discriminative of organic disease. The economic cost of DS arising from the diagnosis of exclusion in CAP was high.
El dolor abdominal crónico (DAC) en la infancia es un motivo de consulta frecuente que afecta a la vida familiar y en ocasiones precisa realización de pruebas complementarias. El objetivo fue realizar el análisis cualitativo, cuantitativo y económico de las pruebas que se solicitan.
Pacientes y métodosEstudio observacional, prospectivo y multicéntrico, incluyendo pacientes entre 4-15 años con DAC. Se diferenciaron dos grupos: orgánico y funcional. Se recogieron las siguientes variables: clínicas, pruebas complementarias y su coste.
ResultadosSe incluyeron 235 niños con DAC (edad media 9,7 ± 2,7 años). Un 79% resultaron trastornos funcionales y un 21% orgánicos. Casi la mitad de los pacientes presentaba algún tipo de síntoma o signo de alarma, pero sólo la clínica miccional se asoció con organicidad. La ecografía abdominal, estudio de parásitos en heces, test de hidrógeno espirado y gastroscopia, son las que más se asociaron con patología orgánica. Existía una diferencia apreciable entre el coste de las pruebas según cada centro. El gasto económico total fue de 52.490,8 euros, siendo 195 euros por paciente para los funcionales y 306 euros para los orgánicos.
ConclusionesLos síntomas y signos de alarma en el DAC son frecuentes, pero poco específicos. La ecografía abdominal y el estudio de parásitos podrían ser pruebas útiles de primer nivel por su inocuidad para diferenciar TO de TDAF. La gastroscopia y el test de hidrógeno espirado fueron las pruebas más discriminativas de organicidad. El coste económico invertido en pruebas para la orientación diagnóstica del DAC de origen funcional es elevado.
Chronic abdominal pain (CAP) is a frequent presenting complaint in children and adolescents at every level of care, accounting for 18%–24% of primary care visits (especially between ages 4 and 12 years). Most cases involve functional disorders, and it is estimated that only 10% have an underlying organic cause.1–3 The pathogenesis of functional gastrointestinal disorders (FGIDs) is not well understood, although it seems to involve the interaction of genetic, environmental and psychosocial factors. These factors promote abnormal reactivity in the enteric, autonomic and central nervous systems in response to physiological changes or stress.2,4–6
The recognition of warning signs and symptoms, a thorough history-taking and physical examination and the duration of symptoms continue to be the gold standard to differentiate organic from functional abdominal pain.2,4,7 The usefulness of diagnostic tests in these diseases continues to be unclear. At the same time, the lack of specific biochemical and structural markers in FGIDs further complicates the diagnosis of affected patients.1,8 All of this, combined with the intensity and frequency of the symptoms and the concern of the family, lead to performance of numerous tests in these children, which corresponds to substantial health care costs.8–10 To these costs we must add the missed work by parents and missed school by children, which occur in up to 85% of cases (mean of 17.6 days a year),11 the loss in quality of life for children and their families and the costs of multiple prescription drugs or other remedies.2,9,12,13
The updated Rome IV criteria are a significant advance in this regard, as they allow clinical diagnosis of FGIDs without diagnostic tests or with use of diagnostic tests for confirmation alone. These criteria also contemplate the possibility that a patient with organic disease (such as coeliac disease or inflammatory bowel disease) may have associated FGIDs. In many cases, the significant anxiety of patients and/or parents may justify performance of diagnostic tests, especially when abdominal pain has a significant impact on the quality of life of the patient.8,9,14,15
Since FGIDs are frequent in the paediatric age group and often lead to performance of multiple diagnostic tests to rule out an organic cause, the primary objective of our study was to perform a quantitative and qualitative analysis of the diagnostic tests ordered in these patients. The secondary objective was to calculate the cost of performed tests.
Sample and methodsWe conducted a prospective, observational multicentre study in 8 Spanish hospitals in the autonomous communities of Valencia, Castilla-La Mancha and Murcia (4 tertiary hospitals and 4 regional hospitals). We considered for inclusion every child aged 4–15 years that made an initial visit to the paediatric gastroenterology clinic due to CAP. We included all who had experienced at least 1 episode of abdominal pain a week in the past 2 months.4 We collected data through a specifically designed application, DOABDO (code no. 09/2014/736), after obtaining parental informed consent. Each physician entered the patient data in the application: age, sex, personal and family history of gastrointestinal disease, characteristics of abdominal pain, presence of warning signs in the anamnesis (pain outside umbilical region, in the right upper or lower quadrant, changes in stools, blood in stools, nausea or vomiting, dysphagia, nocturnal diarrhoea, nocturnal awakening with pain, fever, urethral syndrome, weight loss or deceleration of linear growth) and in the examination (evidence of weight loss, pain on palpation in the right hypochondriac region or right inguinal region, detection of hepatomegaly or rectal anomalies).1,4 We recorded every diagnostic tests performed at the primary care level and then added the tests ordered by specialists. The performed diagnostic tests included blood tests (complete blood count, liver, renal and iron panels; serological tests for coeliac disease, erythrocyte sedimentation rate and C-reactive protein levels), stool tests (occult blood, culture, parasites and calprotectin), urinalysis, abdominal ultrasound, hydrogen test and endoscopy. Last of all, we obtained the costs of the tests performed from the accounting department and calculated the weighted mean for the total. The statistical analysis was performed with the software SPSS® version 18.0, dividing the sample in 2 groups based on the Roma III criteria that were applied at the time of data collection: organic gastrointestinal disorders (OGIDs) and FGIDs. We compared all variables using contingency tables and used the chi square test to study the association between variables and the presence of organic disease. The study was approved by the Ethics Committee of the Hospital Clínico Universitario de Valencia.
ResultsThe sample included 235 children with CAP, 59% female, with a mean age of 9.7 ± 2.7 years and a median age of 10 years. Eighty-five percent were referred from a primary care centre and the rest from a hospital ward, the emergency department or outpatient clinics. Based on the final diagnosis, 79% of patients had FGIDs and 21% OGIDs. Fig. 1 presents the distribution of each group by diagnosis.
When it came to weight status, based on the standards of the World Health Organization (WHO), 74.5% of children had normal weight, 3% underweight and 22.5% excess weight (overweight/obesity), with no differences between the FGID and OGID groups. As for the personal and family history of gastrointestinal disease (Table 1), we found a greater frequency of positive histories in the FGID group, although the difference was not statistically significant.
Association of personal and family history of gastrointestinal disorders with the diagnosis.
| Personal history n (%) | Family history n (%) | |
|---|---|---|
| Functional disorders | 27 (15) | 43 (24.7) |
| Organic disorders | 10 (4.2) | 12 (5) |
| Oesophageal disorder | 0 (0) | 0 (0) |
| Gastric disorder | 2 (25) | 3 (37.5) |
| Intestinal disorder | 7 (14.6) | 9 (18.8) |
| Other | 1 (25) | 0 (0) |
*Chi square test: personal and family history (P = .525 and P < .336, respectively).
We found that warning signs or symptoms were present in nearly half of patients (43.8%), without statistically significant differences between the OGID and FGID groups (61% vs 39%). The presence of pain in the right lower quadrant, pain outside the umbilical region or pain on palpation of the right hypochondriac or inguinal regions was associated with abnormal findings in the abdominal ultrasound scan, although the association was not significant. Diarrhoea occurred in 11.5% of the sample. In the latter group of patients, we identified one case of salmonella infection, but faecal calprotectin levels were normal in all patients but 2, who had values of 287 and 760 mg/g that had normalised in the follow-up test. The only variable significantly associated with organic disease in the chi square test was urethral syndrome (P < .001).
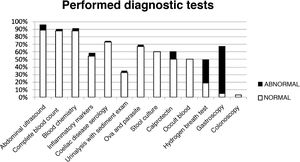
The analysis of the diagnostic tests showed that blood tests had been ordered in 88% of the sample, abdominal ultrasound scans in 88.5%, some form of stool test in 67%, urinalysis with sediment examination in 33% and endoscopic examinations in 8%. Table 2 summarises the results of these tests. Ova and parasite tests, faecal calprotectin levels, hydrogen breath tests and endoscopic examinations were ordered more frequently in the OGID group compared to the FGID group. Fig. 2 presents the distribution of diagnostic tests with the proportions of abnormal and normal results. Of all the tests included in the analysis, only the hydrogen breath test and gastroscopy were significantly associated with the presence of organic disease based on the chi square test (P = .009 and P < .001, respectively).
List of diagnostic tests performed in children with chronic abdominal pain and distribution of results.
| Diagnostic test | Ordered tests n (%) | Abnormal results n (%) | Findings (n) |
|---|---|---|---|
| Abdominal ultrasound | 208 (88.5) | 17 (8) | Renal lithiasis (1) |
| Cholecystitis (2) | |||
| Ileitis (1) | |||
| Mesenteric lymphadenitis (13) | |||
| Complete blood count | 206 (88) | 5 (2.4) | Normocytic anaemia (4) |
| Eosinophilia (1) | |||
| Blood chemistry | 206 (88) | 7 (3.4) | Hypercholesterolemia (3) |
| Ferropenia (2) | |||
| Hypertransaminasaemia (2) | |||
| Anti-transglutaminase IgA antibody | 171 (72.8) | 2 (1.2) | Antibodies > 10 times upper limit of normal |
| Acute phase reactants | 128 (54.4) | 6 (4.6) | None |
| Urinalysis | 78 (33) | 2 (2.5) | Haematuria (1) |
| Cystitis (1) | |||
| Stool parasites | 158 (67.2) | 7 (2.7) | Blastocystis hominis (2) |
| Enterobius vermicularis (3) | |||
| Giardia lamblia (2) | |||
| Stool culture | 141 (60) | 1 (0.7) | Salmonella spp. (1) |
| Faecal calprotectin | 118 (50) | 13 (11) | 50–200 mcg/g stool (11) |
| >200 mcg/g stool (2) | |||
| Hydrogen breath test | 45 (19) | 14 (31) | Fructose malabsorption (12) |
| Fructose malabsorption (2) | |||
| Gastroscopy | 11 (4.7) | 7 (63.3) | Eosinophilic oesophagitis (1) |
| Chronic gastritis (2) | |||
| Gastritis due to Helicobacter pylori (2) | |||
| Duodenal villous atrophy (1) | |||
| Duodenal ulcer (1) | |||
| Colonoscopy | 8 (3.4) | 0 (0) |
Table 3 presents the average cost of each test, with a difference in cost for the same test of up to €200 between participating hospitals based on the autonomous community. The total cost based solely on the diagnostic tests performed in the entire sample under study was of 52 490.8 euros; 64.5% (€33 840) corresponding to tests in the FGID group and the rest (€18 651) to the OGID group. The mean cost of testing per patient with CAP was of €195 in the FGID group versus €306 in the OGID group. We did not take into account the indirect costs in time and human resources.
Average cost in euro of the diagnostic tests in participating hospitals.
| Diagnostic test | Mean (€) | Standard deviation | Range |
|---|---|---|---|
| Complete blood count | 3.1 | 2.1 | 1.82–7 |
| Blood chemistry | 22.1 | 4.6 | 12.08–23.09 |
| Coeliac serology | 45.3 | 25.9 | 4.4–54.14 |
| Urinalysis | 3.8 | 2.4 | 1.08–6.88 |
| Stool ova and parasites | 24.2 | 10.1 | 1.63–24.24 |
| Faecal occult blood | 3.8 | 1.2 | 1.07–4.17 |
| Stool culture | 26.2 | 10.2 | 5.3–26.16 |
| Faecal calprotectin | 35.6 | 16.4 | 3–40 |
| Hydrogen breath test | 45.4 | 6.5 | 40.75–50 |
| Abdominal ultrasound | 58.6 | 18.8 | 21.56–66 |
| Gastroscopy | 242.6 | 82.8 | 104.37–317.52 |
| Colonoscopy | 300.1 | 79.7 | 166.47–351.42 |
Chronic abdominal pain in children is a complaint that generates significant health care use both at the primary care level and in specialised care. Our study is relevant on account of the high prevalence of CAP and FGIDs in our region. A recent meta-analysis that included 58 studies on 200 000 patients reported a prevalence of FGIDs of 13.5% in the worldwide paediatric population,16 with higher rates of 20.7% in patients aged less than 10 years and 26.6% in patients aged 10–18 years reported in a study conducted in the Mediterranean-European region published in 2018.17
The lack of known markers of FGIDs is one of the reasons that these disorders have to be diagnosed based on clinical features. In many cases, this results in the performance of numerous tests to arrive to a diagnosis of exclusion.2,4,7,10
In our sample, we found a prevalence of CAP secondary to organic disease of 21%, greater compared to previous studies that have reported a prevalence of 10%–16%.1,2,15,18 Our figure may overestimate the actual prevalence, as the study was conducted in children that had been referred to a specialist due to either the severity of symptoms or the presence of warning signs. Chronic abdominal pain due to functional disorders resolves in most children, although it persists in up to 30% after 5 years of follow-up, with the best predictors of this outcome being a positive family history, such as presence of gastrointestinal disease in one of the parents (in our cohort, this was the case in one fourth of patients, demonstrating the high frequency of this factor) and detection of relevant genetic or environmental factors.3,8,19 Furthermore, a family history of gastrointestinal disease (especially gastritis in the context of Helicobacter pylori) can play a role through contagion. Parental anxiety at the persistence of the symptoms is another factor that may explain the significant health care demand,1,14 which highlights the importance of physicians establishing rapport with the patient and family and of using simple psychological support and behaviour modification resources to improve the management of FGIDs.
As for warning signs and symptoms, their presence in children with CAP is frequent, reaching up to 80% in some series. Their high prevalence combined with their low discriminatory power for the detection of an organic cause has raised a debate among the experts,1,2,4 and the level of evidence is not sufficient to support their use. The absence of warning signs and symptoms is highly specific for diagnosis of FGIDs, but with a low sensitivity.1,18,21 In agreement with the previous literature, warning signs and symptoms did not predict the presence of organic disease in our sample, with the exception of urethral syndrome, for which the association was statistically significant.
As concerns diagnostic tests, the complete blood count and acute phase reactants are very nonspecific, and in our sample they were not useful as the initial screening for organic disease, in agreement with the findings of other authors.8,10,18 Tests to screen for coeliac disease, as described in the literature, do not offer a good yield in the initial evaluation of CAP unless it is associated with diarrhoea.1,18,21,22 We ought to highlight that in our sample serological tests for coeliac disease were positive in 2 cases (2/171), with an incidence that was close to 1%–2% found in the larger population.
When it came to the abdominal ultrasound, a subject that has not been clarified to date, we found as many as 8.1% of scans with abnormal findings, in contrast to other case series in which the proportion was of less than 1%. Although comparing data is difficult, since this is a rater-dependent test, it is known that the frequency of abnormal sonographic findings increases to approximately 10% in patients presenting with warning signs such as urethral syndrome, back pain, vomiting etc.1,23 Many authors dispute the relevance or yield of abdominal ultrasound. We consider that this is a useful diagnostic method. When it came to stool tests, there were pathological findings in 2.7% of the ova and parasites tests ordered in our cohort. Infections by protozoans such as Giardia lamblia or Blastocystis hominis are considered to cause CAP among other manifestations. Enterobiasis (Enterobius vermicularis) can also cause pain, usually accompanied by other local manifestations. Due to the sociodemographic and epidemiological changes in Spain, the parasites involved in these infections are shifting and the incidence on the rise.24 Given our findings, we consider that the ova and parasites test, along with the abdominal ultrasound, can be included in the initial evaluation of CAP, as these tests are noninvasive and inexpensive.8,23 Faecal calprotectin is a simple test that can be very helpful in screening for organic disease. As described in the literature, it is a sensitive but nonspecific marker that can be used to identify patients that may require an endoscopic examination to rule out inflammatory bowel disease and prevent performance of this test in patients with FGIDs.23,25,26 In our study, there were only 2 tests with results exceeding 200 mg per g of stools in patients in which the levels subsequently normalised. In the past, the American Academy of Paediatrics Subcommittee on Chronic Abdominal Pain (2005) recommended performance of a faecal occult blood tests in children with CAP, without warning signs or symptoms and with a normal physical examination.1,8 This recommendation has become obsolete with the successive updates of the Roma criteria on account of the better yield of faecal calprotectin. Our findings support this change, as all the tests for occult blood ordered in our sample turned out negative. Lastly, gastroscopy and the hydrogen breath test were the tests with the highest specificity for detection of an organic aetiology in our study, as a diagnosis was made in cases with compatible manifestations and warning signs and symptoms.
Analysing the costs associated with the care of children with CAP is important, as they amount to 13.7% of the total per capita health care budget in Spain (data from 2015).27 This is due to the high prevalence of CAP, the substantial health care demand associated to it and the considerable number of diagnostic tests performed. We found a striking difference in the cost of diagnostic tests within Spain between autonomous communities, with differences of up to €200 for one type of test. Overall, the cost of the diagnostic tests ordered in patients with FGIDs was nearly double the cost of the tests used in patients with OGIDs (€33 840 vs €18 651), mainly on account of the greater prevalence of FGIDs. In contrast, when we analysed the health care cost per patient, we found that it was higher in the OGID group compared to the FGID group (€306 vs €195). This is probably due to the generally significantly higher cost of the tests performed in children with suspected organic disease, such as endoscopy, compared to other tests. One of the limitations of our study was that we studied the cost of diagnostic tests without taking into account human resources and time.16 In this sense, a study conducted in the Netherlands that analysed the direct medical costs excluding diagnostic tests and indirect costs of care due to losses of parental paid work estimated a cost of €2512 a year per child with functional abdominal pain or irritable bowel syndrome.28 It is difficult to establish these indirect costs in the Spanish public health system, especially in comparison with studies conducted in the United Sates, where this calculation is more feasible on account of its multi-payer/private health care system.29 A study in a sample of 243 children with CAP in the United States calculated costs of $6104.30 per patient and $744 726 overall, significantly higher compared to our study.10 A study published by Lane et al.29 comparing the costs derived from the diagnosis of 89 children with FGIDs based on whether they had been managed by paediatric gastroenterologists or by primary care physicians found that the costs in the first group were 5–9 times greater compared to those in children managed at the primary care level. However, our results show that the cost per patient with organic disease was only 1.5 times higher compared to the cost per patient with a functional disorder. This is probably due to the high prevalence of FGIDs, which overall are 20 times as frequent as OGIDs. A recent study of the costs associated with the care of patients with functional disorders estimated an expenditure of up to 16 billion dollars in adults.13,20 Longstreth et al. analysed health care expenditure in relation to the severity of symptoms, finding that expenditure increased by 35%, 52% and 59% with the presence of mild, moderate and severe symptoms, respectively.30 It would be useful to identify the strengths and weaknesses of each level of care in Spain to use resources more efficiently in the management of these patients.
In conclusion, CAP is a frequent presenting complaint in the paediatric age group both at the primary care and specialty care levels. As observed in our sample, a family history of functional disorders is very frequent in patients with FGIDs, probably due to the psychosocial and genetic factors involved in their pathogenesis. In addition, warning signs and symptoms are frequently present in patients with CAP, but they offer little discriminatory power for the identification of organic disease, and it is their absence that has more relevance in diagnosis. As for the different diagnostic tests, based on our findings we consider that the abdominal ultrasound scan and stool ova and parasites test could be useful in the initial evaluation to differentiate organic from functional disorders, as they are harmless. Ultrasound allowed ruling out many organic diseases with a noninvasive approach, although it would be preferable if this result could be corroborated in studies with larger samples. Gastroscopy and the hydrogen breath test offered the highest specificity. The costs of diagnostic tests used for the diagnosis by exclusion of FGIDs is high, and we should be aware of the substantial expenditure that they entail and try to be selective of the tests that we order based on the suspected diagnosis.
Conflicts of interestThe authors have no conflicts of interest to declare.
Previous presentations: This study was presented at the XXIII National Congress of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica, May 12–14, 2016, Gijón, Spain; the 50th Annual Meeting of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition; May 10–13, 2017, Prague, Czech Republic; and the XXV National Congress of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica, May 17–19, 2018, Granada, Spain.
Please cite this article as: Jiménez Candel MI, Salvador Pinto T, García Peris M, Crehuá Gaudiza E, Jovaní Casano C, Moreno Ruiz MA, et al. Rendimiento de las pruebas complementarias en el estudio de pacientes con dolor abdominal crónico. An Pediatr (Barc). 2021;95:26–32.