Patent ductus arteriosus (PDA) is a prevalent condition in preterm infants, and may be related to increased morbidity and mortality in the most immature newborns. Recent studies have examined the usefulness of brain natriuretic propeptide (proBNP) in the diagnosis of this pathology. The aim of the study was to evaluate the diagnostic efficacy of proBNP as a marker of haemodynamic overload in PDA.
Paients and methodsA retrospective study was conducted on preterm infants less than 32 weeks of gestation and/or weight less than 1500g. Echocardiogram and determination of proBNP levels were performed on all patients. Comparison was made by subgroups according to the presence of PDA and their haemodynamic characteristics.
ResultsOf the 60 patients enrolled, 71.7% had PDA, of which 86% had haemodynamically significant patent ductus arteriosus (HS-PDA). All of them, but one, received medical treatment with ibuprofen or acetaminophen. Surgical closure was required in 29.7% of HS-PDA.
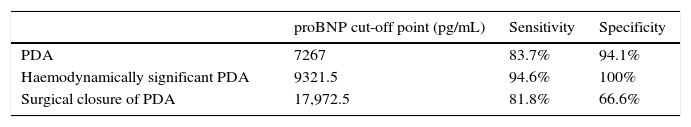
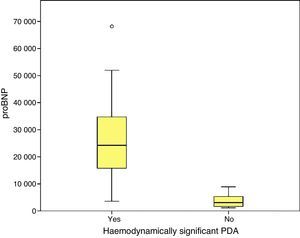
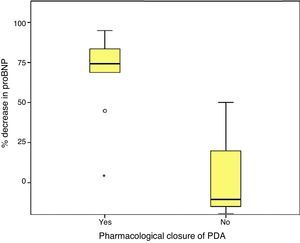
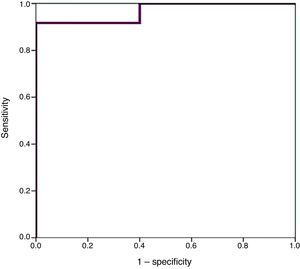
Higher values of proBNP were found in patients with HS-PDA (33,338±34,494.47pg/mL; p=.000) compared to patients with closed or non-haemodynamically significant ductus arteriosus. Higher values were also found in patients who required surgical closure of PDA (30,596.8±14,910.9; p=.004). A greater decrease inproBNP levels was found in the group of patients which duct closure after pharmacological treatment (68±24.69% vs −12.22±99.4%; p=.030). ProBNP cutoff-level for HS-PDA was calculated by ROC curve and it was 9321.5pg/mL (Specificity: 100%, Sensitivity: 94.6%).
ConclusionsProBNP levels are related to the presence or absence of haemodynamically significant patent ductus arteriosus; and its variations with treatment response. High values are also related to the need for surgical closure of PDA.
El ductus arterioso persistente (DAP) es una patología muy prevalente en el recién nacido pretérmino (RNPT), que puede relacionarse con mayor morbimortalidad en los prematuros más inmaduros. Estudios recientes han valorado la utilidad del propéptido natriurético cerebral (proBNP) en su diagnóstico. El objetivo fue evaluar la eficacia diagnóstica del proBNP como marcador de sobrecarga hemodinámica en el DAP y su capacidad para identificar la necesidad de tratamiento.
Pacientes y métodosEstudio retrospectivo observacional, que incluyó a RNPT menores de 32 semanas de gestación y/o 1.500g, con estudio ecocardiográfico y determinación de niveles de proBNP. Se comparó por subgrupos en función de la presencia de DAP y sus características hemodinámicas.
ResultadosDe los 60 pacientes incluidos, el 71,7% presentó DAP, el 86% de los cuales fue hemodinámicamente significativo (DAP-HS). Todos, salvo uno, recibieron tratamiento médico con ibuprofeno o paracetamol. El 29,7% de los DAP-HS precisó cierre quirúrgico.
Se encontraron valores superiores de proBNP en los pacientes con DAP-HS (33.338±34.494,47pg/mL; p=0,000), respecto a los pacientes con ductus cerrado o no hemodinámicamente significativo. Los pacientes que precisaron cirugía también presentaron valores más elevados (30.596,8±14.910,9pg/mL; p=0,004). El grupo en el que se constató cierre ductal tras tratamiento farmacológico presentó mayor descenso de los niveles de proBNP (68±24,69% vs. –12,22±99,4%; p=0,030). Mediante curva ROC se calculó valor de corte de proBNP para el diagnóstico de DAP-HS que fue de 9.321,5pg/mL (E 100%, S 94,6%).
ConclusionesLos niveles de proBNP se relacionan con la presencia o ausencia de ductus persistente hemodinámicamente significativo y sus variaciones con la respuesta al tratamiento. Valores elevados también se relacionan con la necesidad de cirugía.
The ductus arteriosus (DA) is a blood vessel that connects the aorta with the pulmonary artery during foetal life, shunting blood from the pulmonary to the systemic circulation. In most full-term newborns, the DA closes within three days from birth, but in preterm newborns (PTNBs) with birth weights of less than 1500g or born before 32 weeks’ gestation, the incidence of patent ductus arteriosus (PDA) is high, and inversely proportional to gestational age and weight at birth. Approximately 20% of neonates born before 32 weeks’ gestation have DAP, as do more than 50% of extremely low birth weight newborns (<1000g).1,2
The onset of symptoms depends on the size of the left-to-right shunt through the PDA and the ability of the neonate to handle the resulting volume overload, which tends to be less the more immature the newborn.
Preterm newborns with haemodynamically significant PDA (hsPDA) may not develop clinical symptoms, and if they do, it usually starts from 2 to 3 days of life, as they start to recover from respiratory distress. However, PTNBs treated with surfactant may develop symptoms earlier because of the decrease in pulmonary vascular resistance that results from its administration.3,4
Due to the delayed onset of clinical symptoms, several authors have proposed routine screening by echocardiography at 48–72h from birth in very low birth weight PTNBs.5,6
Haemodynamically significant PDA can cause significant morbidity and mortality in the most immature PTNBs (especially those born before 28 weeks’ gestation with weights of less than 1000g), manifesting with symptoms of decreased cardiac output, exacerbating respiratory distress due to pulmonary hyperperfusion and increasing the risk of diseases such as necrotising enterocolitis (NEC), intraventricular haemorrhage (IVH), retinopathy of prematurity (ROP), renal failure (RF) with oliguria or even, although it remains a subject of controversy, bronchopulmonary dysplasia (BPD).7,8 Although this is a widely debated subject, the current approach in many neonatal units is to manage PDA with conservative measures and only use pharmacological treatment in patients in which the ductus is considered haemodynamically significant based on the clinical manifestations or echocardiographic features.9–12
There is also no clear consensus as to the echocardiographic criteria that define a hsPDA, although the most widely accepted criteria at present are a ductal diameter of more than 1.5mm, a retrograde diastolic flow in the descendent aorta of at least 30%, significant enlargement of the left atrium (with a left atrial/aortic root ratio of more than 1.4) or a ductal velocity of less than 2m/s in Doppler ultrasound.9
Surgical treatment is reserved for patients in which pharmacological treatment fails or is contraindicated.13,14
The most widely used criteria to establish the indication of pharmacological treatment of PDA are respiratory status, hypotension with a greater reduction in diastolic blood pressure and echocardiographic findings suggestive of haemodynamic significance. However, there are times when echocardiographic features do not offer a clear assessment of its haemodynamic significance, and to date there are no reliable biomarkers to support the presence of absence of hsPDA that could supplement echocardiographic findings in the decision-making process.
Several recently published studies have proposed the use of pro-brain natriuretic peptide (proBNP) as a biochemical marker of hsPDA. These studies have demonstrated that measurement of serum proBNP levels in the first days of life may be useful in the assessment of haemodynamic compromise and therapeutic decision making.15,16 However, it is not clear whether changes in serial proBNP levels would be useful to predict PDA closure in response to treatment.17,18
Natriuretic peptides are a family of structurally similar but not genetically related peptides that play a significant role in the regulation of renal, cardiovascular and endocrine homeostasis, and there is evidence of a strong enough association of its serum levels with the degree of left ventricular dysfunction, the severity of symptoms and the filling velocity of cardiac chambers in the context of heart failure.19
In this study, we analysed the contribution of proBNP levels to the diagnosis of PDA and the assessment of haemodynamic compromise in PTNBs delivered before 32 weeks’ gestation and/or with birth weights of less than 1500g.
Materials and methodsWe conducted a retrospective observational study with descriptive and inferential analysis that included PTNBs with birth weights of less than 1500g and/or born before 32 weeks’ gestation admitted to the neonatal intensive care unit of the Hospital Infantil Miguel Servet between June 1, 2013 and January 31, 2015. From the date that the study started, levels of proBNP were measured in our unit with every echocardiographic assessment of PDA.
The study included a total of 60 PTNBs delivered before 32 weeks’ gestation and/or with birth weights of less than 1500g.
The protocol for PDA screening in our unit includes performance of an echocardiographic assessment in the first 48–72h of life in all newborns less than 28 weeks’ gestational age and/or with weights of less than 1000g, and in newborns with a greater gestational age or birth weight in which PDA is suspected due to clinical manifestations (worsening of haemodynamic or respiratory status with increased need for ventilatory support, audible murmur on auscultation, metabolic acidosis) or prenatal or postnatal risk factors for PDA, such as volume overload, absence of maturation with corticosteroid treatment, sepsis or maternal diabetes. A blood sample was also collected prior to initiating treatment for a complete blood count and measurement of levels of electrolytes, creatinine and proBNP at the time of the echocardiographic assessment, which was performed in every instance using an ALOKA ProSound Alpha7 system (Hitachi®) by a paediatric cardiologist that was blinded to the results of the proBNP test. Samples were tested with the NT-proBNP kit (Roche Diagnostic®) and results reported in pg/mL. The addition of the proBNP test did not require drawing a larger volume of blood compared to the samples drawn for routine chemistry panels.
In our hospital, pharmacological treatment of PDA consists of intravenous ibuprofen (3 doses every 24h, the first one of 10mg/kg, and the second and third of 5mg/kg), and the alternative treatment for cases in which ibuprofen is contraindicated (patients with thrombocytopaenia, NEC, IVH, active haemorrhage or renal failure) is intravenous paracetamol (for 5 days at doses of 15mg/kg every 6h).
Subsequently, we performed serial measurements of proBNP levels at the same time as the follow-up echocardiographic assessments. We conducted follow-up echocardiographic assessments in patients that had received treatment within 24h from the completion of each course of medication to determine whether a new course of treatment was needed in case the hsPDA criteria persisted, or to take on a watchful waiting approach if the ductus had closed or remained open but did not fulfil the criteria for haemodynamic compromise.
The diagnosis of hsPDA was based on previously established echocardiographic criteria.9
We excluded PTNBs that did not undergo the echocardiographic and laboratory workup (patients that died before undergoing the PDA screen or were born after 28 weeks’ gestation with weights of more than 1000g and without clinical manifestations suggestive of PDA), patients that had other serious heart diseases that could cause volume overload, patients with echocardiographic features of pulmonary hypertension that contraindicated pharmacological treatment at the time of echocardiographic assessment, and patients with clinical sepsis at the time of the echocardiographic and laboratory workup. We defined clinical sepsis as the presence of elevated markers of infection accompanied by symptoms such as changes in body temperature, vomiting, irritability, tachypnoea or tachycardia and recurrent episodes of apnoea. The reason we made sepsis an exclusion criterion is that levels of proBNP may be elevated in patients with systemic infection in the absence of PDA.20
The study protocol was approved by the Clinical Research Ethics Committee of Aragón.
We obtained the data by reviewing the medical records of the patients, collecting data for variables the antenatal and immediate postnatal periods (type of pregnancy [singleton or multiple], sex, reason for delivery, administration of antenatal corticosteroids, gestational age, anthropometric measurements at birth, Apgar score, resuscitation in the delivery room, endotracheal administration of surfactant), neonatal outcome variables (ventilatory and haemodynamic support, development of BPD, NEC, IVH, RF, late-onset sepsis, ROP and death) and findings of echocardiographic assessment, head ultrasound and laboratory workup (complete blood count, electrolytes, creatinine and serial proBNP levels).
Statistical analysisWe entered the data in a database and analysed them using the SPSS software version 21.0.
We performed a descriptive analysis to determine frequencies, central measures of tendency and measures of dispersion. For the inferential analysis, we used the Kolmogorov–Smirnov and the Shapiro–Wilk tests to verify the normality of quantitative variables, and compared these variables between subgroups by means of the Mann–Whitney U test (two independent samples), Wilcoxon test (two related samples) and the Kruskal–Wallis test (several independent samples). We compared qualitative variables by means of the chi square test and Fisher's exact test.
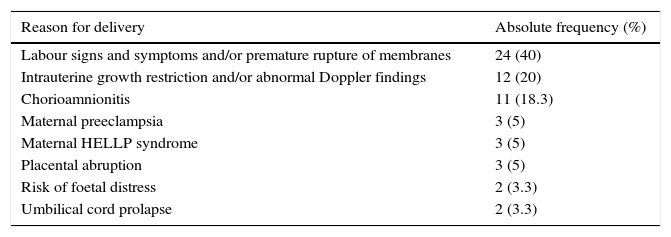
ResultsWe obtained a sample of 60 patients with a mean gestational age of 27.88±2.18 weeks and a mean birth weight of 954.22±271.20g. Tables 1 and 2 show the descriptive characteristics of the sample and the causes of preterm birth.
Cause of preterm birth.
| Reason for delivery | Absolute frequency (%) |
|---|---|
| Labour signs and symptoms and/or premature rupture of membranes | 24 (40) |
| Intrauterine growth restriction and/or abnormal Doppler findings | 12 (20) |
| Chorioamnionitis | 11 (18.3) |
| Maternal preeclampsia | 3 (5) |
| Maternal HELLP syndrome | 3 (5) |
| Placental abruption | 3 (5) |
| Risk of foetal distress | 2 (3.3) |
| Umbilical cord prolapse | 2 (3.3) |
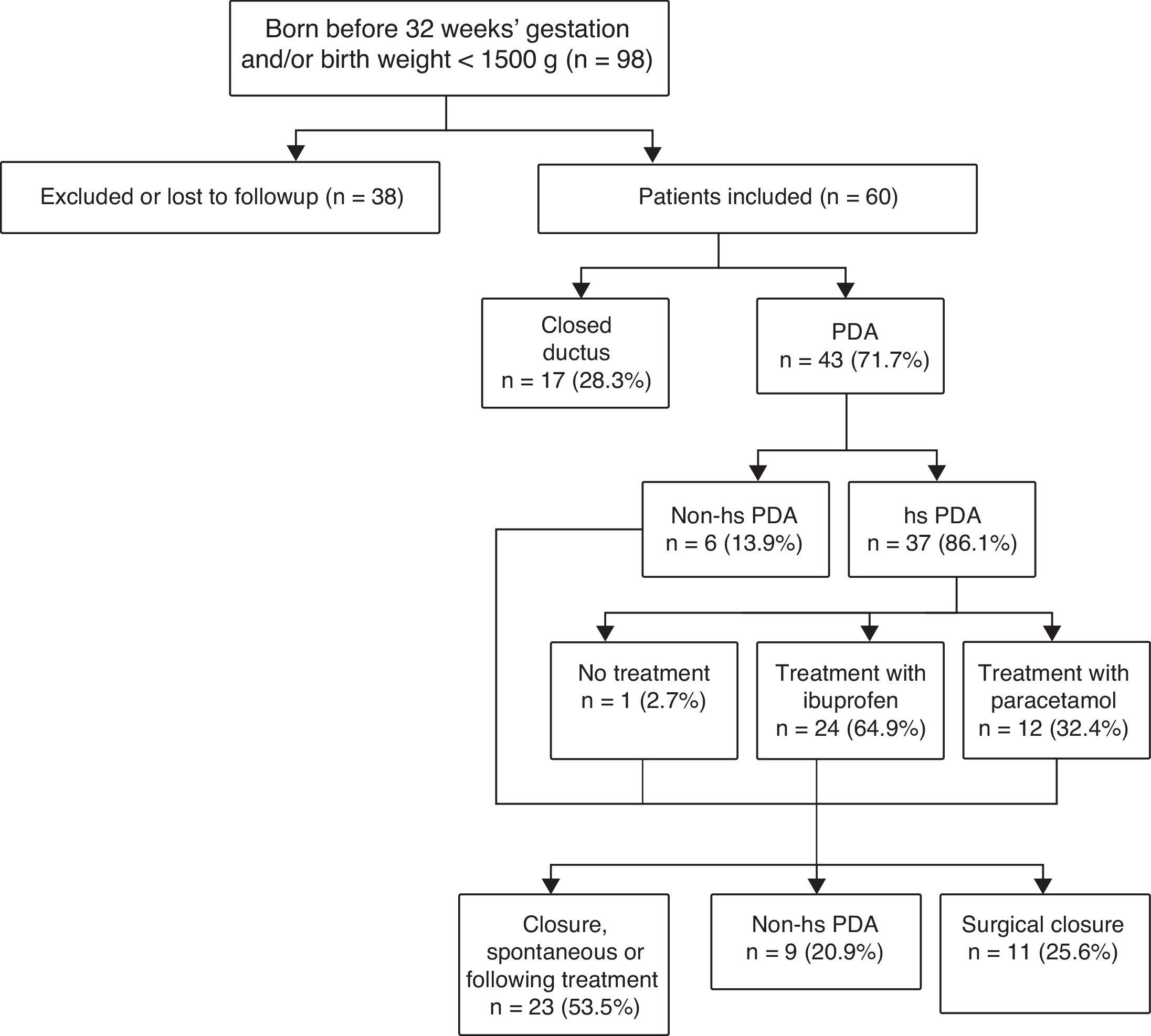
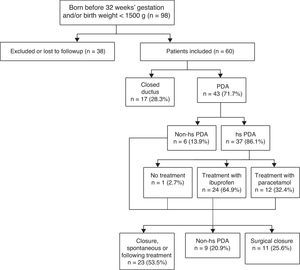
Fig. 1 shows the algorithm used for PDA diagnosis and management.
Of the patients that were born before 28 weeks’ gestation and/or with birth weights of less than 1000g, 75.6% had PDA (85.3% of them had hs-PDA).
We divided the sample into two subgroups, the first one corresponding to patients with hsPDA (n=37) and the second to patients without hsPDA (n=23), with the latter group including patients that had PDA that was not haemodynamically significant as well as patients with a closed ductus at the time of the first echocardiographic assessment.
We did not find any differences in the immediate postnatal variables with the exception of weight, which was lower in patients with hsPDA (902.35±288.29g versus 1037.65±222.36g; p=.018).
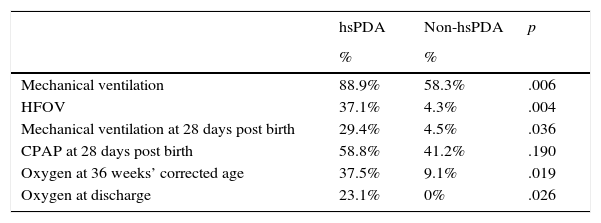
We found statistically significant difference between groups in the need for ventilatory support, which was more frequent in the hsPDA group (Table 3).
Need for ventilatory support in patients with and without hsPDA.
| hsPDA | Non-hsPDA | p | |
|---|---|---|---|
| % | % | ||
| Mechanical ventilation | 88.9% | 58.3% | .006 |
| HFOV | 37.1% | 4.3% | .004 |
| Mechanical ventilation at 28 days post birth | 29.4% | 4.5% | .036 |
| CPAP at 28 days post birth | 58.8% | 41.2% | .190 |
| Oxygen at 36 weeks’ corrected age | 37.5% | 9.1% | .019 |
| Oxygen at discharge | 23.1% | 0% | .026 |
CPAP, continuous positive airway pressure; HFOV, high-frequency oscillatory ventilation.
We did not find a statistically significant association between proBNP levels and the gestational age and weight at birth.
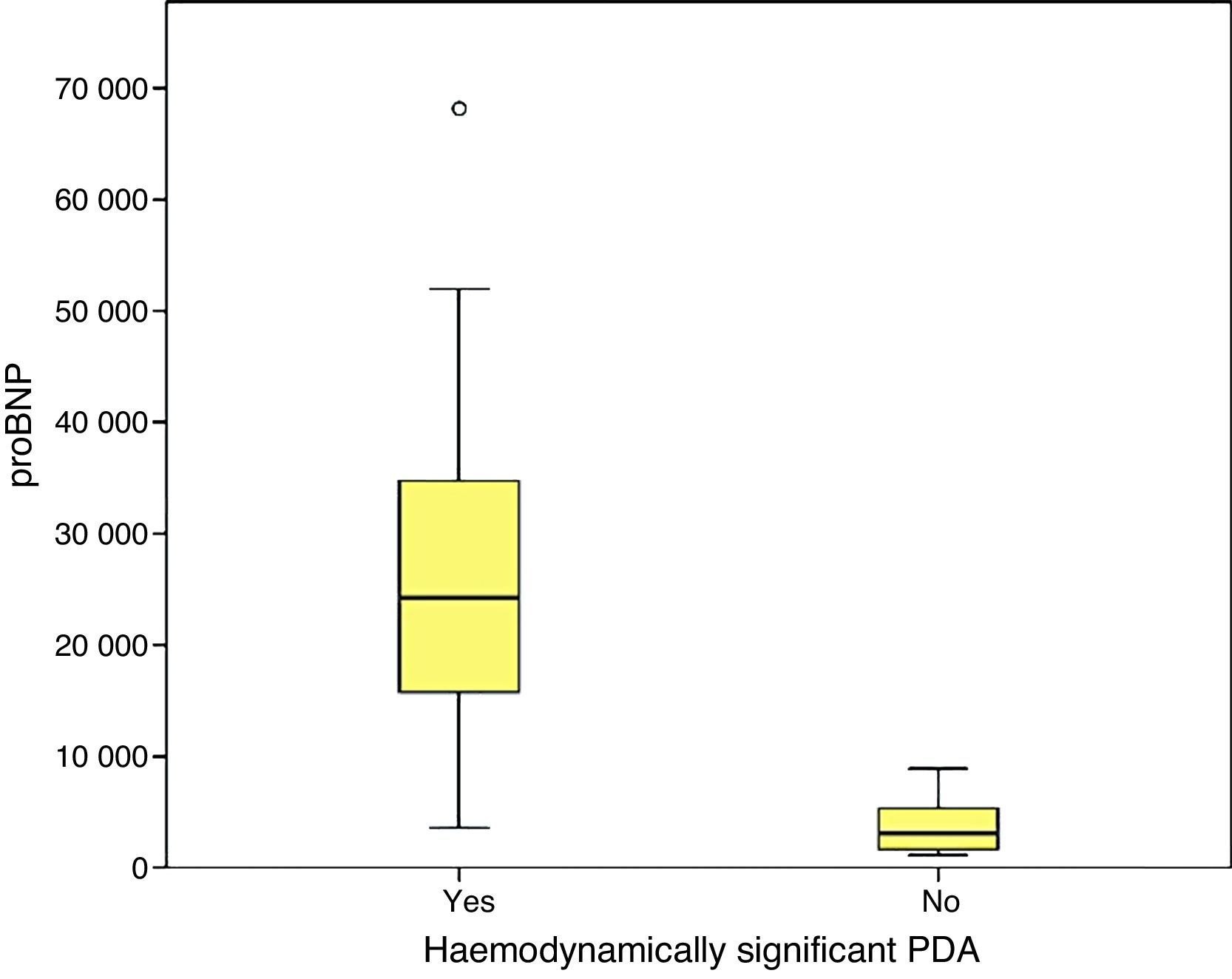
We found statistically significant differences in the levels of proBNP between PTNBs with PDA and newborns with a closed ductus in the initial echocardiography (29,773±33,778.74pg/mL versus 3722.5±2669.76; p=.000) and between newborns with hsPDA and newborns without hsPDA (33,338.1±39,325.6pg/mL versus 3390.7±2338.3pg/mL; p=.000). In the subgroup of PTNBs without hsPDA, a total of six patients had PDA with no echocardiographic signs of haemodynamic compromise, and their proBNP levels were 4387.1±2260.2pg/mL, with a statistically significant difference relative to the hsPDA group (p=.000).
The baseline proBNP levels were also significantly higher in PTNBs that eventually required surgical closure (30,596.82±14,910.94pg/mL versus 19,221.13±32,891.21pg/mL; p=.004).
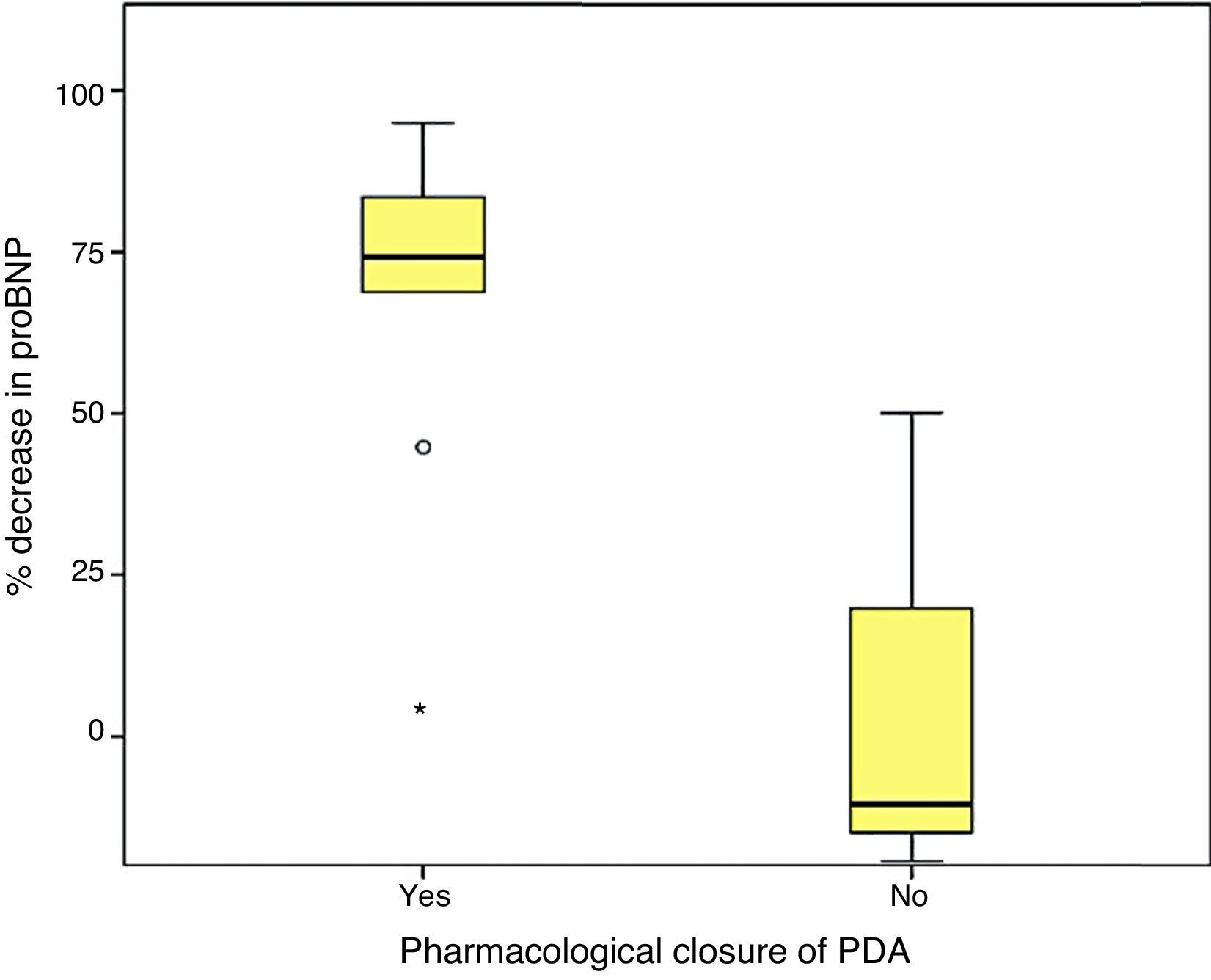
Last of all, we found that the percent decrease in levels of proBNP was significantly higher in the group that had achieved ductal closure with pharmacological treatment, (68%±24.69% versus −12.22%±99.4%; p=.030) (Figs. 2 and 3).
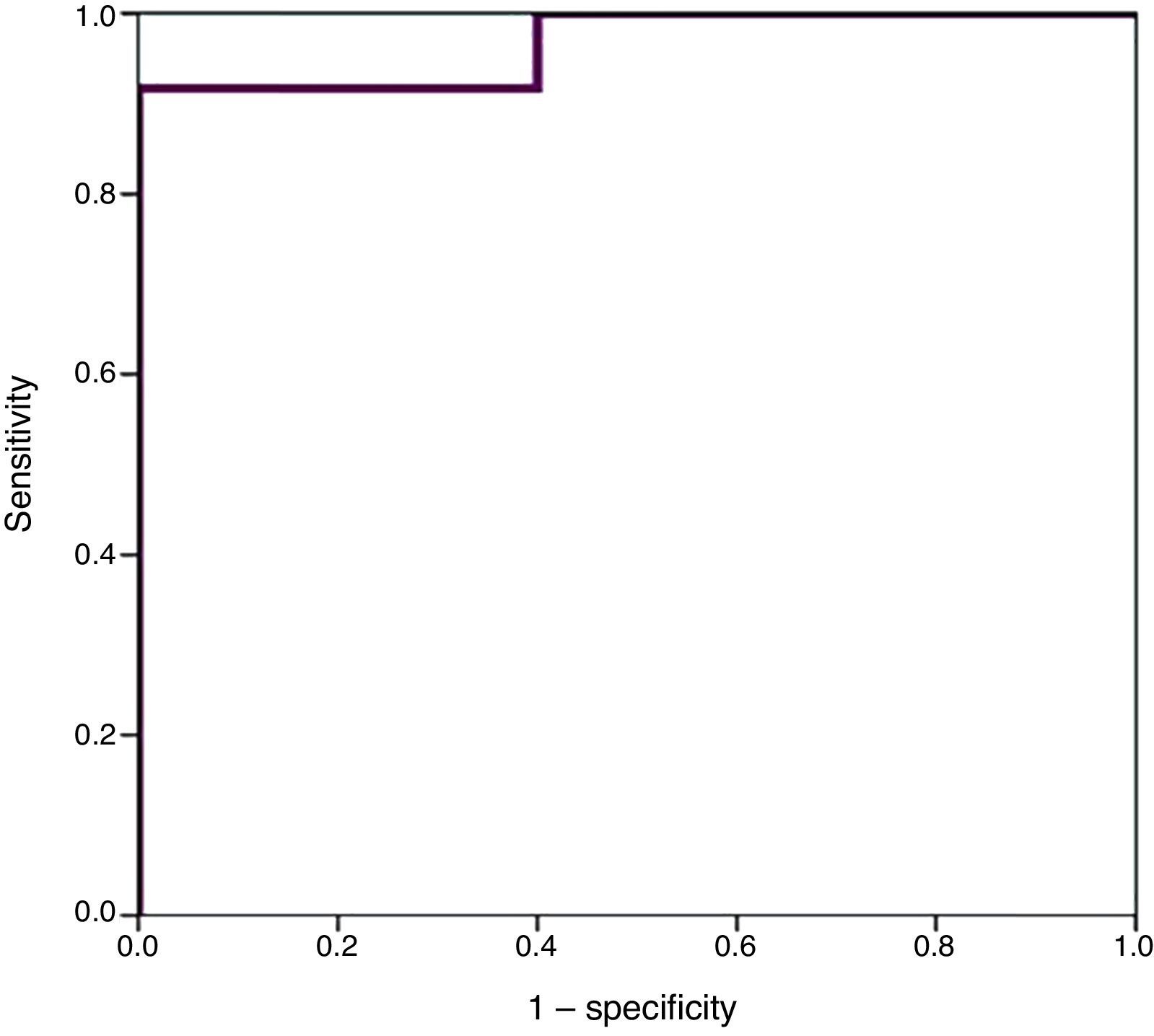
We used ROC curves to determine the optimal cut-off points in this sample for the diagnosis of PDA, hsPDA and the risk of requiring surgical closure (Table 4 and Fig. 4).
Patent ductus arteriosus is a disease with a high prevalence in PTNBs that increases in inverse proportion to birth weight and gestational age.
It can cause significant morbidity and mortality in the most immature newborns, increasing the risk of developing certain diseases characteristic of this population, and it has even been speculated that it carries a higher risk of BPD, although this remains a controversial hypothesis.7,8,21 Consequently, the strategies to achieve its closure are still being debated, since the optimisation of PDA management through the selection of patients eligible for treatment is probably key to reduce the associated morbidity and mortality without treating patients that do not require it, given its adverse effects.12,22
The debate concerning the appropriate diagnostic criteria and ideal timing for the treatment of PDA continues today, and there are still no universally accepted guidelines for its management. Thus, approaches to the management of PDA have been changing, evolving from strategies that are being phased out, such as prophylactic treatment in the first days of life, to current strategies based on the treatment of PTNBs with haemodynamically significant PDA.9,13,21,23
In our sample, 71.7% of patients had features of PDA in the initial ultrasound, a percentage that was higher than others reported in the literature, with a prevalence of PDA of 50% in PTNBs of less than 32 weeks’ gestational age and/or with birth weights of less than 1500g,24 although prevalences of up to 60% or 70% have been found in other series.25 In our study, the prevalence we found was influenced by the exclusion of newborns that did not undergo the initial echocardiographic assessment, which had the most impact on the 28-to-32 weeks’ gestational age group.
Of the patients born before 28 weeks’ gestation and/or with weights of less than 1000g, 75.6% had PDA (which was haemodynamically significant in 85.3% of them).
In our study, we found an association between proBNP levels in the first 48–72h of life and the presence of PDA, which, considering what has been described by other authors,26,27 seems to suggest that proBNP levels are a reliable parameter for predicting the presence or absence of PDA.
Furthermore, we found hsPDA in 61.7% of patients, who had higher proBNP levels than patients in the group without hsPDA (which included patients with PDA that was not haemodynamically significant and patients with a closed ductus arteriosus). When we limited the comparison to patients with PDA, we still found that proBNP levels could be used to discriminate patients with haemodynamic compromise from those without. Using a ROC curve, we established an optimal cut-off point of 9321.50pg/mL for the prediction of hsPDA in our sample, which had a sensitivity of 91.7% and a specificity of 100%, meaning that in our sample, serum proBNP levels above this point were indicative of hsPDA, and could be used to complement echocardiographic findings to determine the need for treatment. Furthermore, the cut-off point had an optimal sensitivity, so that few patients with hsPDA had levels below the cutoff and remain unidentified. Based on these findings, our study confirms that serum proBNP levels are a reliable predictor of the presence of hsPDA. In line with these findings, PDA has been treated in other populations based on the measured levels of this biomarker without awaiting the results of the echocardiographic assessment,17,27,28 confirming that only a small percentage of patients with hsPDA are left untreated with this approach, and that few patients are treated unnecessarily. In any case, we think it is prudent to not rely solely on the levels of proBNP to determine the need for treatment, and that echocardiography continues to be the gold standard for the diagnosis of PDA. The measurement of proBNP levels should be considered a supplement to the echocardiographic examination.
On the other hand, consistent with the findings of other series,17,27 we found a fairly strong association between the levels of proBNP and PDA closure, which was predicted reliably by significant drops in proBNP levels.29
Furthermore, we found that the baseline proBNP levels in our sample were associated with an increased incidence of surgical closure of PDA,17 although in this case the clinical applicability in predicting the need for surgery was limited due to the low sensitivity and specificity values, and the considerable controversy surrounding this option results in a significant variability between different neonatal units.
In conclusion, the findings of our study confirmed those of previous publications18,19,26–28 that reported an association between the levels of proBNP and haemodynamic overload secondary to PDA in PTNBs. This biomarker is associated with the presence or absence of haemodynamically significant PDA and its changes in response to treatment.
The presence of elevated proBNP levels can supplement echocardiographic findings in the assessment of the need for pharmacological treatment, and declines in these levels are indicative of an adequate response to the treatment.
Although echocardiography is and will continue to be the gold standard for the diagnosis of PDA, the findings of our study support the use of proBNP as a supplemental tool for the diagnosis and followup of PDA, helping guide therapeutic decision-making.
In any case, further prospective studies with larger sample sizes are needed to establish more reliable cut-off points for the levels of proBNP in relation to this disease.30
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Montaner Ramón A, Galve Pradel Z, Fernández Espuelas C, Jiménez Montañés L, Samper Villagrasa MP, Rite Gracia S. Utilidad del propéptido natriurético cerebral en el diagnóstico y manejo del ductus arterioso permeable. An Pediatr (Barc). 2017;86:321–328.
Previous presentation: This study was presented as an oral communication at the 63rd Congress of the Asociación Española de Pediatría; June 2015; Bilbao, Spain. Honourable Mention Award in the best oral communications category at the 63rd Congress of the Asociación Española de Pediatría; June 2015; Bilbao, Spain.