To evaluate the prevalence of non-approved prescriptions (off-label and unlicensed) in a neonatal intensive care unit (NICU), and to describe factors of the neonate associated with its use.
Materials and methodsObservational prospective study in a level III NICU during a 6-month period. Every prescription was analysed using the summary of product characteristics as a reference. A sequential algorithm was used to create a classification of prescriptions based on current status: approved, unlicensed, off-label (by age, route of administration, dosage, or indication).
ResultsThe study included 84 patients and 564 prescriptions. A total of 127 (22.5%) prescriptions were considered off-label, and 45 (8%) were considered unlicensed. More than half (59.5%) of the patients received at least one of these drugs, and this increases to 100% among very preterm neonates and surgical patients (P<.001). A positive linear correlation was found between duration of NICU stay and the number of off-label prescriptions (correlation coefficient 0.6; P<.001).
ConclusionsNon-licensed drugs are frequently prescribed in NICU, especially in the most vulnerable patients. Our results show the need to move forward on clinical research in order to homogenise the existing data about neonatology drugs, with the aim of making an efficient and safe prescription.
Evaluar la prevalencia de prescripciones en condiciones no autorizadas (off-label y unlicensed) en una Unidad de Cuidados Intensivos Neonatales (UCIN) y definir qué características de los neonatos se asocian a un mayor uso de fármacos en estas condiciones.
Material y métodosEstudio observacional prospectivo en una UCIN nivel III-C durante un periodo de 6 meses. Se evaluó la condición de uso de cada fármaco, tomando como referencia su ficha técnica. Se utilizó un algoritmo secuencial para la clasificación de las prescripciones en: aprobadas, unlicensed u off-label (por edad, por indicación, por vía de administración, y por dosis).
ResultadosSe incluyeron 84 pacientes y 564 prescripciones. Un total de 127 prescripciones fueron consideradas off-label y 45 unlicensed; lo cual supuso el 22,5% y el 8% del total, respectivamente. El 59,5% de los pacientes recibieron al menos un fármaco en una de estas condiciones, ascendiendo este porcentaje al 100% en los grandes prematuros y en los pacientes quirúrgicos (p<0,001). Se encontró una correlación lineal positiva entre la estancia en UCIN y el número de prescripciones off-label (coeficiente de correlación 0,6 p<0,001).
ConclusionesLa prescripción de fármacos en condiciones no autorizadas es habitual en UCIN, siendo especialmente frecuente en los pacientes con mayor vulnerabilidad. Estos resultados ponen de manifiesto la necesidad de avanzar en la investigación y homogeneizar la información existente sobre los fármacos en neonatología, con el objetivo de realizar una prescripción eficaz y segura.
Newborns are a particularly vulnerable group, especially those that need intensive care, who also happen to be those whose management requires the most drugs.1–3 In spite of this, the use of drugs in newborns is often not supported by clinical trials specific to this population, and is rather based on the extrapolation of safety and efficacy data from trials in adults or paediatric case series.4–6
Special access to medicines is regulated by Royal Decree 1015/2009 under three different circumstances: use in research, access to drugs marketed in other countries and use under conditions other than those established in the marketing authorisation.7 The latter includes off-label use (outside the indications specified in the summary of product characteristics) and the use of unlicensed (not authorised) drugs under certain conditions, such as modifications of authorised drug formulations or the use of compounded medicines; both are particularly frequent in paediatrics,8,9 but specific studies on the neonatal population are scarce. The off-label prescription of drugs under unauthorised conditions is important from a legal as well as a clinical standpoint, as it is associated with an increased risk of adverse events in both children and adults.10,11
We designed a prospective observational study whose primary objective was to assess the prevalence of unauthorised prescription (UP) in a neonatal intensive care unit (NICU). The secondary objective was to assess what characteristics of newborn patients were associated with the off-label and unlicensed use of medicines.
Materials and methodsWe conducted a prospective observational study at the level IIIC NICU (the highest level of care based on the standards of the Sociedad Española de Neonatología [Spanish Society of Neonatology]) of a public university hospital in Spain.
The period under study encompassed 6 consecutive months (April to September 2018), with collection of data on all patients admitted to the NICU whose parents or legal guardians signed the informed consent form. We achieved 100% participation, and the final sample included 564 prescriptions of 85 different drugs in 84 patients. This sample size allowed us to estimate the prevalence of UP with a confidence of 95% and a precision of ±4% for the total population of newborns managed in the NICU. We collected data for all the variables under study in 2 data collection notebooks, and the source of the data was the electronic health records of the patients.
We defined off-label and unlicensed prescription based on the summaries of product characteristics approved by the Agencia Española del Medicamento y Productos Sanitarios (Spanish Agency of Medicines and Medical Devices [AEMPS]) and/or the European Medicines Agency (EMA).12 In cases where the summary of product characteristics was not available or the information it provided was ambiguous, we consulted other databases, chiefly Pediamecum® (of the Committee on Medicines of the Asociación Española de Pediatría [Spanish Association of Paediatrics])13 and Botplus® (of the Consejo General de Colegios Farmacéuticos [General Council of Boards of Pharmacists]), based on which the research team reached a consensus on how to classify the drug.
Based on a previous consensus,14 we defined unlicensed use as use of a drug not covered by a marketing authorisation as medicinal for human use. We also decided, as was done in previous studies,15 that this category included the use of compounded formulations prepared in hospital. We defined off-labeluse as use of a drug already covered by a marketing authorisation, in an unapproved way (taking into account the indication, age group, route of administration and dosage). To classify prescriptions, we used a sequential algorithm that has been published in the past.15
We classified each drug according to the Anatomical Therapeutic Chemical Classification system (ATC) of the World Health Organization.16 For the purpose of the study, we did not include as drugs any blood products, enteral or parenteral nutrition, oxygen or fluids.
For each drug subject to off-label or unlicensed use, we determined whether there was a current paediatric investigation plan under the EMA (a development plan approved by the EMA Paediatric Committee for the purpose of collecting the necessary data to support the authorisation of a medicine for paediatric use by means of studies designed specifically for this population).
Before starting the study, we filed for registration of the study by the AEMPS and obtained the approval of the competent research ethics committee.
We performed a descriptive analysis of all the variables, summarising quantitative variables as mean±standard deviation and median, and qualitative variables as absolute frequencies and percentages. We calculated the prevalence of UP with the corresponding 95% confidence intervals (CIs). We assessed the association between qualitative variables by means of the chi square test. We compared the means of 2 groups with the Mann–Whitney U test after verifying the normal distribution of the data by means of the Kolmogorov–Smirnov test, and compared the means of more than 3 groups using the de Kruskal–Wallis test. We analysed the association between quantitative variables by means of the Spearman correlation coefficient. We defined statistical significance as a P-value of less than 0.05 in two-tailed tests. All the statistical analyses were performed with the software package SPSS version 19.0.
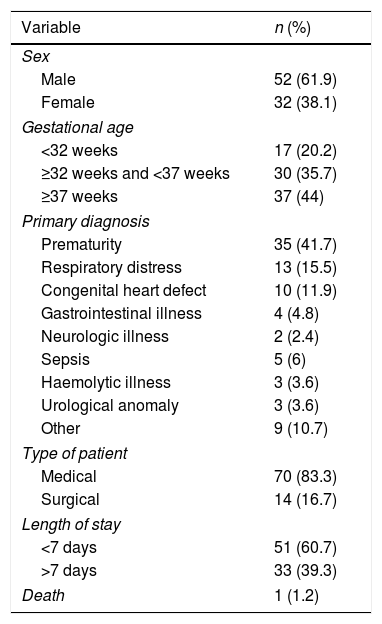
ResultsWe analysed 564 prescriptions of 85 drugs in 84 patients. Table 1 presents the baseline characteristics of the patients.
Descriptive analysis of the patients, n=84.
| Variable | n (%) |
|---|---|
| Sex | |
| Male | 52 (61.9) |
| Female | 32 (38.1) |
| Gestational age | |
| <32 weeks | 17 (20.2) |
| ≥32 weeks and <37 weeks | 30 (35.7) |
| ≥37 weeks | 37 (44) |
| Primary diagnosis | |
| Prematurity | 35 (41.7) |
| Respiratory distress | 13 (15.5) |
| Congenital heart defect | 10 (11.9) |
| Gastrointestinal illness | 4 (4.8) |
| Neurologic illness | 2 (2.4) |
| Sepsis | 5 (6) |
| Haemolytic illness | 3 (3.6) |
| Urological anomaly | 3 (3.6) |
| Other | 9 (10.7) |
| Type of patient | |
| Medical | 70 (83.3) |
| Surgical | 14 (16.7) |
| Length of stay | |
| <7 days | 51 (60.7) |
| >7 days | 33 (39.3) |
| Death | 1 (1.2) |
The mean number of prescriptions per patient was 6.7±6.3 (median, 4). The minimum number of prescriptions per patient was 1, and the maximum was 43.
The most frequently prescribed drugs (including unauthorised and authorised prescriptions) were calcium gluconate (12.4% of total prescriptions), oral calcium (8.7%), ampicillin (7.6%) and gentamicin (7.1%).
Based on the conditions under which drugs were used, 127 of prescriptions (22.5%; 95% CI, 19–26%) corresponding to 35 drugs were off-label. The mean number of off-label prescriptions per patient was 1.5±2.4 (median, 1). On the other hand, 45 prescriptions (8%; 95% CI, 5.6–10.3%) of 15 drugs were unlicensed. The mean number of UPs per patient was 0.6±1 (median, 0). Thus, 59.5% of the patients (n=50; 95% CI, 48.4–70.6%) was given a prescription under conditions other than those authorised (57.1% at least 1 off-label prescription and 32.1% at least one unlicensed prescription).
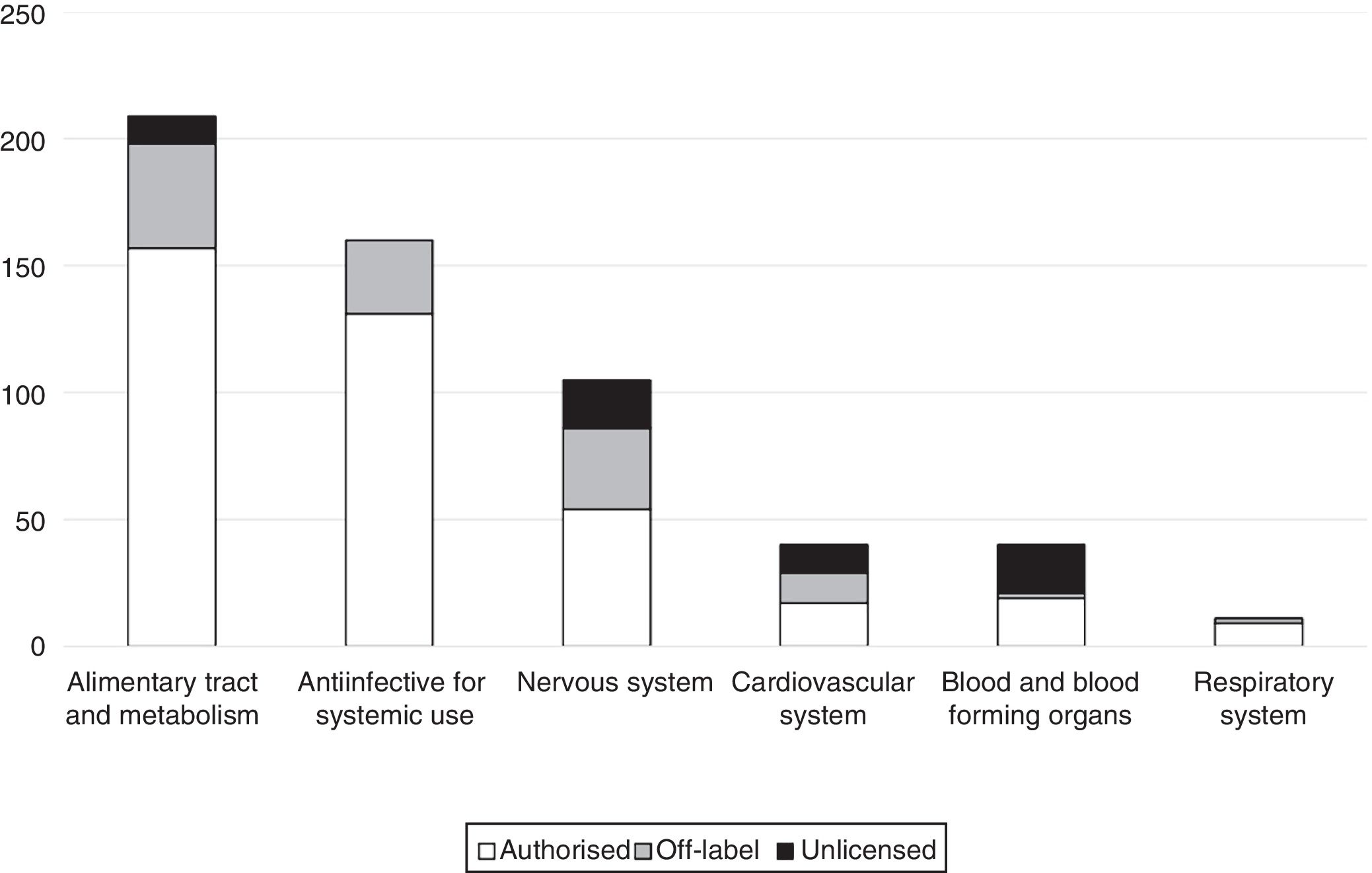
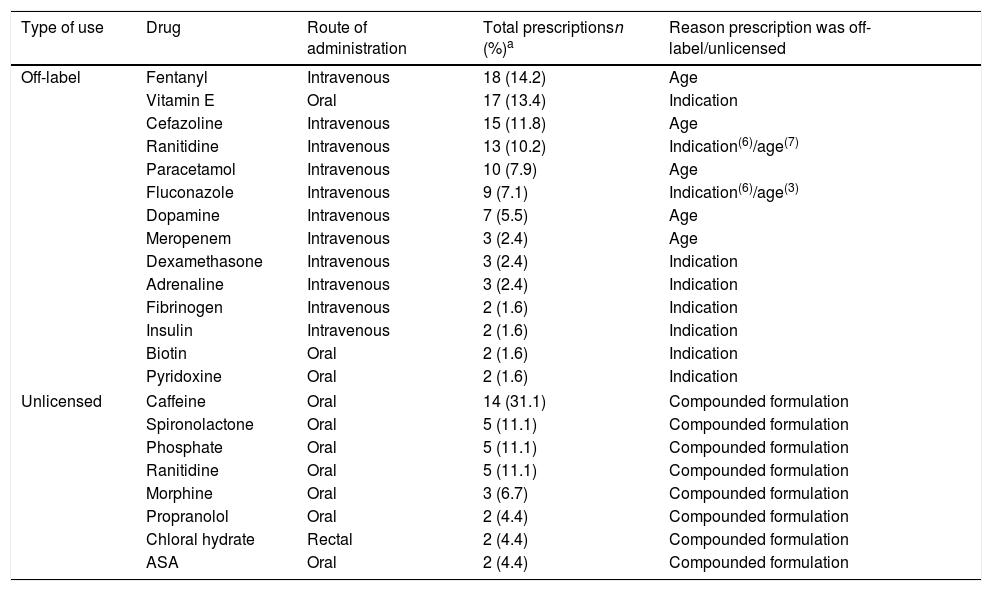
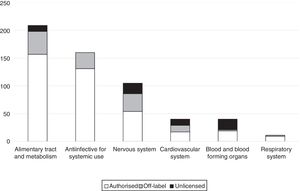
The main reason why prescriptions were off-label was the age of the patient (55.1%), followed by the indication (41.7%). Only 3 prescriptions were off-label due to the prescribed dosage (2.4%); and only 1 due to the route of administration (0.8%). Table 2 presents the drugs most frequently prescribed under off-label or unlicensed conditions. Fig. 1 presents the distribution of off-label prescriptions by ATC group. We found that 34.6% of off-label prescriptions and 20% of unlicensed prescriptions were for drugs for which the EMA had approved a paediatric investigation plan.
Drugs most frequently used in the NICU by type of use.
| Type of use | Drug | Route of administration | Total prescriptionsn (%)a | Reason prescription was off-label/unlicensed |
|---|---|---|---|---|
| Off-label | Fentanyl | Intravenous | 18 (14.2) | Age |
| Vitamin E | Oral | 17 (13.4) | Indication | |
| Cefazoline | Intravenous | 15 (11.8) | Age | |
| Ranitidine | Intravenous | 13 (10.2) | Indication(6)/age(7) | |
| Paracetamol | Intravenous | 10 (7.9) | Age | |
| Fluconazole | Intravenous | 9 (7.1) | Indication(6)/age(3) | |
| Dopamine | Intravenous | 7 (5.5) | Age | |
| Meropenem | Intravenous | 3 (2.4) | Age | |
| Dexamethasone | Intravenous | 3 (2.4) | Indication | |
| Adrenaline | Intravenous | 3 (2.4) | Indication | |
| Fibrinogen | Intravenous | 2 (1.6) | Indication | |
| Insulin | Intravenous | 2 (1.6) | Indication | |
| Biotin | Oral | 2 (1.6) | Indication | |
| Pyridoxine | Oral | 2 (1.6) | Indication | |
| Unlicensed | Caffeine | Oral | 14 (31.1) | Compounded formulation |
| Spironolactone | Oral | 5 (11.1) | Compounded formulation | |
| Phosphate | Oral | 5 (11.1) | Compounded formulation | |
| Ranitidine | Oral | 5 (11.1) | Compounded formulation | |
| Morphine | Oral | 3 (6.7) | Compounded formulation | |
| Propranolol | Oral | 2 (4.4) | Compounded formulation | |
| Chloral hydrate | Rectal | 2 (4.4) | Compounded formulation | |
| ASA | Oral | 2 (4.4) | Compounded formulation | |
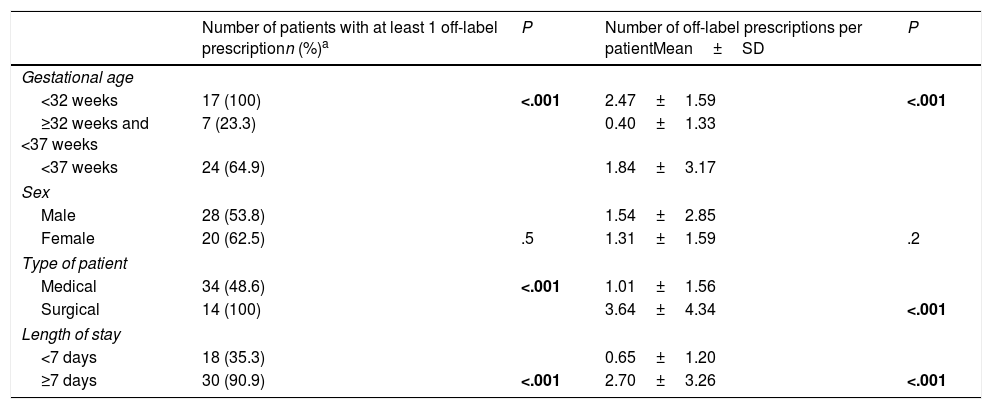
Table 3 presents the results of the bivariate analysis of the factors associated with off-label prescription. We found a significant association between being a surgical patient, being born very preterm or a length of stay of 7 days or longer and a greater frequency of off-label prescription. We did not find differences in the frequency of off-label prescription based on sex. We also found a positive linear correlation between the length of stay in the NICU and the frequency of off-label prescription (correlation coefficient, 0.6; P<.001).
Analysis of off-label prescriptions based on patient characteristics.
| Number of patients with at least 1 off-label prescriptionn (%)a | P | Number of off-label prescriptions per patientMean±SD | P | |
|---|---|---|---|---|
| Gestational age | ||||
| <32 weeks | 17 (100) | <.001 | 2.47±1.59 | <.001 |
| ≥32 weeks and <37 weeks | 7 (23.3) | 0.40±1.33 | ||
| <37 weeks | 24 (64.9) | 1.84±3.17 | ||
| Sex | ||||
| Male | 28 (53.8) | 1.54±2.85 | ||
| Female | 20 (62.5) | .5 | 1.31±1.59 | .2 |
| Type of patient | ||||
| Medical | 34 (48.6) | <.001 | 1.01±1.56 | |
| Surgical | 14 (100) | 3.64±4.34 | <.001 | |
| Length of stay | ||||
| <7 days | 18 (35.3) | 0.65±1.20 | ||
| ≥7 days | 30 (90.9) | <.001 | 2.70±3.26 | <.001 |
SD, standard deviation.
Our study evinced that prescription of drugs under unauthorised conditions (off-label and unlicensed prescription) is frequent in the critically ill neonatal population, as more than half of newborns in our sample received at least one prescription under conditions other than those authorised. This percentage reached 100% in the subsets of very preterm newborns and surgical patients.
The unauthorised use of a drug does not only hinder its understanding and clinical application, but also increases the risk of adverse events. This is the case in the paediatric population, with an increased in the relative risk of adverse events of 3.44 compared to authorised prescription,10 as well as in adults.11 This effect is probably also present in the neonatal population. Although few studies in the literature focus on the highly specific population of critically ill newborns, all of them report that the use of drugs under unauthorised conditions is quite frequent in these patients. In the different studies on the subject, the proportion of off-label prescriptions ranged between 12% and 87%; and the proportion of unlicensed prescriptions between 4% and 19%.5,6,17–29
The method employed in each study to categorise a prescription as off-label is not an insignificant factor, and it is probably one of the main reasons for the considerable variability of the results of studies that are apparently similar. In 2017, García-López et al. published a study in a sample of 42 critical paediatric patients in Anales de Pediatría where they applied a sequential algorithm to categorise prescriptions based on the factor that determined their off-label or unlicensed condition.15 This compelling study is what inspired us to make a similar analysis of data in the neonatal population, so we decided to use the same stepwise scheme to categorise drug prescriptions in our sample.
Our criteria to classify off-label prescriptions based on age were probably less stringent compared to previous studies. Lass et al.19 (Estonia, 2011), Neubert et al.22 (Germany, 2010) and Dell’Aera et al.27 (Italy, 2007) defined as authorised prescriptions of drugs whose summary of product characteristics explicitly authorised their use in newborns or preterm newborns, as applicable, and otherwise considered prescriptions to be off-label based on the age of the patient. In contrast, we only considered a prescription to be off-label based on age if the summary of product characteristics explicitly specified that use of the drug was contraindicated in newborns or gave specific directions for use by age group and did not include the neonatal age group, whereas if it referred to the “paediatric age group” in general without further distinction we considered its use in newborns authorised.
Another aspect to consider is that if we had only taken into account the AEMPS summary of product characteristics for the classification of prescriptions, the percentage of unauthorised prescriptions would have been higher, as the AEMPS database (CIMA) does not include summaries of product characteristics for some of the drugs that were prescribed frequently. In other instances, the information given in the summaries of product characteristics of the AEMPS was ambiguous, which compelled us to consult other databases (such as Pediamecum® or Botplus®) and, in some cases, to decide on the classification of prescriptions by consensus of the research team. To offer an example of a controversial prescription in our study, the summary of product characteristics of furosemide solution for injection of the AEMPS states, verbatim, that “in infants and children aged less than 15 years, parenteral administration of furosemide (in some instances, slow intravenous infusion) will only be performed in cases where the life of the patient is at risk”. We found the specification of “the patient's life being at risk” ambiguous, as patients that received this drug were not at risk of imminent death if they were not given it, but they did need the drug in some way to have a favourable outcome in the context of a critical condition; under these circumstances, our team considered this type of use to be authorised prescription. Another example was the use of amikacin, for which the summary of product characteristics of the AEMPS includes neonatal sepsis as an authorised indication, whereas the Pediamécum® explicitly states that its use should always be considered off-label in newborns. In accordance with the summary of product characteristics, we considered its use authorised, but we ought to highlight the presence of contradictory information in 2 databases that are widely used in everyday clinical practice.
These instances and other cases that we have not described here attest to the considerable challenge we faced to categorise drug prescriptions based on the conditions of use. After comparing our study to similar works, we found that the scheme applied for this classification has an impact on the results obtained in research. We believe that this is an important limitation of our study and any other similar investigation that may be conducted in our field, but at the same time it is a very relevant finding, as it evinces the need to standardise summaries of product characteristics with an emphasis of providing information concerning the paediatric population—and the neonatal population in particular—in a clearer and better organised manner.
At the international level, studies generally describe higher proportions of unauthorised prescriptions. The studies published by Avenel et al. (France)6 and Conroy et al. (United Kingdom),23 both from 1998, reported proportions of 64% of off-label and 10% of unlicensed prescriptions and 55% of off-label and 10% of unlicensed prescriptions, respectively. Since two decades separate their publication from our study, it is difficult to compare their results to ours. On the other hand, a lower overall gestational age in the samples of other studies is another factor that may have had an impact in the higher frequency of unauthorised prescriptions reported by their authors.6,20,24,25 For instance, O’Donnell et al. (Australia, 2001)20 found that 47% of prescriptions were off-label and 11% unlicensed, but the median gestational age of their patients was 31 weeks, compared to 35+6 weeks in our study.
As for Spain, in 2016 Blanco-Reina et al.30 published the results of a study conducted in a paediatric and neonatal intensive care unit in Granada, reporting 52% of off-label prescriptions out of a total sample of 601 prescriptions to 81 patients. In 2017, as we already mentioned, García-López et al.15 analysed 696 prescriptions to 42 patients in the paediatric intensive care unit of a hospital in Madrid and found proportions of 54% (off-label) and 9% (unlicensed). The main difference compared to our sample was that these studies included patients aged up to 14 and 18 years, which are not comparable to our patients, as our study had a sample consisting exclusively of newborns. A study by Arocas-Casañ et al.29 from 2017 (NICU of the Hospital de Murcia, 41 patients and 273 prescriptions) found 41% of off-label prescriptions and 5.5% of unlicensed prescriptions, although there were some differences compared to our study as to which prescriptions were considered unauthorised.
A common finding in most studies is that the drugs used most frequently under off-label conditions belong to the anti-infective group,17–19,22,23,26,29 but in our study the greatest proportion corresponded to the alimentary tract and metabolism group (32% of off-label prescriptions). This finding is probably due to the significant percentage contributed by drugs such as vitamin E (which is widely used in newborns and was categorised as off-label in our sample due to its indication) and ranitidine (also frequently used in newborns and categorised as off-label due to the indication or the age of the patient depending on the circumstances), both of which belong to this group. Another group corresponding to drugs whose use is frequently considered unauthorised is the group of drugs acting on the central nervous system,17,20,22,26 which also corresponded to a large percentage in our study (1/4 of the total number of off-label prescriptions), only inferior to the alimentary tract and metabolism group.
A salient aspect we ought to highlight is the higher frequency of unauthorised prescription in the most vulnerable patients, such as very preterm newborns (already described in previous studies18,22) or patients that undergo a major surgery during hospitalisation, a frequent phenomenon in neonatal care, where the most critically ill patients are also the ones who receive the riskiest treatments.
Overall, the studies that we reviewed provided a general perspective on the issue of unauthorised drug prescription in newborns. The differences in the reported results probably stem from the heterogeneity of the studies due to factors mentioned above (differences in the criteria for categorisation of prescriptions as unauthorised, the gestational age of patients or the historical context, among others), although we also ought to consider the variability associated with geographical location, which largely determines which summaries of product characteristics are consulted and which medical conditions are most prevalent in the patients under study (prematurity in France6,24 [as was the case in our study], hyperbilirubinemia in Estonia19 and respiratory distress and sepsis in India26).
LimitationsThere are limitations to our study. The main one is the lack of a universal standard for the classification of off-label drug use, which we discussed above, and above all the lack of concrete information in the summaries of product characteristics of many drugs regarding the indications, age groups and dosages approved for their use. Another limitation is that we conducted the study in a single neonatal unit, which also had certain characteristics that may further differentiate it, such as the greater presence of patients with complex congenital heart defects, as our hospital is the reference centre for neonatal cardiac surgery (which means that these patients, who have a higher risk profile, may have been overrepresented in our study, which could be a source of bias). On the other hand, our study was intended as an initial exploration of the prevalence of off-label prescription, and as such we did not assess the adverse events associated with unauthorised prescription nor whether these prescriptions could have been replaced by others under authorised conditions, aspects that may be relevant and that we intend to take into account in future studies.
ConclusionsA substantial percentage of the prescriptions issued in neonatal intensive care are unapproved (off-label or unlicensed), which means that more than half of the patients receive treatments under these conditions, something that is even more frequent in the most vulnerable populations, such as very preterm newborns. We think that it is essential that we clarify and consolidate the information currently available on the use of medicines in the neonatal population and monitor adverse events, with establishment of high-quality registers, to be able to offer treatments of guaranteed safety and effectiveness within an appropriate ethical and legal framework.
Conflicts of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: Alonso AS, Avila-Alvarez A, Eiriz MC, Roca CM, Gómez PY, López AC, et al. Uso de medicamentos en condiciones no aprobadas en cuidados intensivos neonatales. An Pediatr (Barc). 2019;91:237–243.