The use of high-flow cannula therapy (HFNC) in neonatal units has increased in recent years, but there are no consensus guidelines on its indications and application strategies. Our aim was to know the rate of use of HFNC, their indications and the management variability among Spanish neonatal units.
Material and methodsTwenty-five-question survey for medical and nursing staff. Level II and III units were contacted by phone and sent in Google forms between September 2016 and December 2018.
ResultsNinety-seven responses (63.9% medical, 36.1% nursing), from 69 neonatal units representing 15 autonomous communities (87% level of care III; 13% level II). All units except one have HFNC with a humidified and heated system. Their most frequent indications are: non-invasive ventilation weaning (79.4%), bronchiolitis (69.1%), respiratory distress of the term newborn (58.8%), after extubation (50.5%). Minimum flow (1–5 L/min) and maximum flow (5–15 L/min) are variable between units. 22.7% have experienced some adverse effect from its use (9 air leak, 12 nasal trauma).
Less than half have an employment protocol, but all the answers agree on the usefulness of national recommendations.
ConclusionsHFNC therapy is widely used in Spanish units, but there is great variability in its indications and strategies of use. National recommendations would be applicable in most units and would allow unifying its use.
El uso de la terapia con cánulas de alto flujo (CNAF) en las unidades neonatales ha experimentado un incremento en los últimos años, pero no existen guías de consenso sobre sus indicaciones y estrategias de aplicación. Nuestro objetivo fue conocer la tasa de empleo de CNAF, sus indicaciones y la variabilidad de uso entre las unidades neonatales españolas.
Material y métodosEncuesta de 25 preguntas dirigida a personal médico y de enfermería. Se contactó telefónicamente con unidades de nivel II y III y se envió en formato Google Forms entre septiembre 2016 y diciembre 2018.
ResultadosSe recibieron 97 respuestas (63,9% medicina, 36,1% enfermería), de 69 unidades neonatales que representan a 15 comunidades autónomas (87% nivel asistencial III; 13% nivel II). Todas las unidades, salvo una, disponen de CNAF con sistema humidificado y caliente. Sus indicaciones más frecuentes son: destete de ventilación no invasiva (VNI) (79,4%), bronquiolitis (69,1%), distrés respiratorio del recién nacido a término (RNT) (58,8%), tras extubación (50,5%). El flujo mínimo varía entre 1–5 lpm y el flujo máximo entre 5–15 lpm. El 22,7% ha experimentado algún efecto indeseado por su uso (9 fuga aérea, 12 traumatismo nasal).
Menos de la mitad tiene protocolo de empleo, pero todas las respuestas coinciden en la utilidad de unas recomendaciones nacionales.
ConclusionesLa terapia con CNAF está ampliamente extendida en las unidades españolas, pero existe gran variabilidad en sus indicaciones y estrategias de utilización. Unas recomendaciones a nivel nacional serían aplicables en la mayoría de las unidades y permitirían unificar su empleo.
Respiratory disease is one of the main causes of morbidity and mortality in neonatal intensive care units. One of the factors most clearly associated with the development of chronic lung disease is the duration of invasive mechanical ventilation.1 As a result, new non-invasive ventilation (NIV) strategies have grown in importance in recent years, such as high-flow nasal cannula (HFNC) therapy.
The use of HFNC seems to improve the efficiency of breathing by flooding the nasopharyngeal space with a constant high flow, contributing to decreasing the work of breathing and to the clearance of CO2.2,3 Studies in animals and humans have demonstrated that high flow rates can generate a certain degree of positive end-expiratory pressure (PEEP) both in the airway and the pharynx.2,3 While this pressure is not stable and cannot be controlled, it also does not seem hazardous if it is delivered in adherence with recommendations that the cannula occupy less than 50% of the nostril diameter. High flow oxygen therapy offers advantages such as the ease of use, improved comfort and tolerability for the patient and acceptance by health professionals and families. All of the above has promoted its widespread use in neonatal care units,4–9 despite the lack of solid scientific evidence supporting this modality for some indications.10–12
The main indications for use of high-flow oxygen therapy in neonatal patients at the moment are: as an alternative to respiratory support with continuous positive airway pressure (CPAP) post extubation in newborns delivered at or after 28 weeks’ gestation and as an alternative to CPAP for weaning from respiratory support in clinically stable preterm infants at risk of nasal trauma.13 The advantages in relation to CPAP evinced by different studies include the simplicity of the interface, the ease of its set up, the lower incidence of nasal trauma or air leaks, patient comfort, family satisfaction and improved mother-child bonding.14
However, there are no protocols or consensus-based guidelines at the national or international level defining indications and implementation strategies to standardise its management. This makes it difficult to guarantee optimal respiratory care in these patients and hinders the performance of multicentre studies with an adequate sample size that would allow more accurate establishment of the role of high-flow oxygen therapy in the neonatal population.
The main objective of our study was to establish the frequency of use of HFNC in neonatal care units in Spain in addition to which patients it is used in, its indications and how it is delivered. We also analysed differences in its use based on the level of care,15 the devices used for its delivery, the perception of the care team of its advantages and disadvantages and the need to standardise its management at the local and regional levels.
Material and methodsAfter reviewing the current literature on the use of HFNC in neonatal care, a questionnaire was developed by a multidisciplinary team formed by medical and nursing staff of our neonatal unit with expertise in respiratory support.
The questionnaire comprised 25 items with multiple choice, Yes/No or open-ended answer options (Table 1).
Items in the national survey on the use of high-flow nasal cannulae in neonatal patients.
| Questionnaire items |
|---|
| 1. Facility |
| 2. Professional category* |
| 3. Annual birth volume |
| 4. Neonatal patients managed in the unit (based on gestational age and weight) |
| 5. Supportive care systems available in the unit (conventional mechanical ventilation, parenteral nutrition, high-frequency oscillatory ventilation, nitric oxide, neonatal extracorporeal membrane oxygenation) |
| 6. Availability of HFNC in the unit * |
| 7. Type of neonatal patients for who HFNC are used* |
| 8. How do you think high-flow oxygen works?* |
| 9. Indications for use of HFNC in the unit* |
| 10. Availability of a protocol/guideline for use of HFNC in the unit* |
| 11. Which HFNC device is available in the unit?* |
| 12. What are the characteristics of the HFNC device in the unit? (heated gas, humidified gas, both, neither)* |
| 13. Flow used to initiate therapy in the unit* |
| 14. Minimum flow rate used* |
| 15. Maximum flow rate used* |
| 16. Are different interface sizes available?* |
| 17. Do you think that a protocol and specific training for the use of HFNC need to be developed?* |
| 18. If so, which aspects of this modality seem most relevant and should be addressed in specific training?* |
| 19. How is the use of HFNC perceived in your unit?* |
| 20. What is your personal opinion?* |
| 21. What are the benefits you think it may offer to the newborn?* |
| 22. What are the disadvantages it may have for the newborn? |
| 23. Has your unit experienced any adverse events associated with the use of HFNC? If so, what adverse effects have been observed in your unit?* |
| 24. Do you think that the development of national guidelines for the use of HFNC would be useful?* |
| 25. If there were guidelines for the use of HFNC at the national level, do you think they would be applicable to your unit?* |
Starting from an initial list of neonatal units in public and private hospitals, we selected hospitals with level II and level III units, defined based on the criteria established in the document on the levels of care and recommended minimum requirements for neonatal care of the Sociedad Española de Neonatología.15 This classification is based on the complexity of the diseases managed in the unit and the annual birth volume of the facility.
We telephoned the units to request contact information for a point person in respiratory care among the nursing and the medical staff. We then called the identified individuals to explain the objectives of the survey, after which we submitted the questionnaire through the Google Forms platform.
Between September 2016 and December 2018, we submitted the questionnaire to 230 professionals in 120 units. We were unable to obtain a valid email address for 10 professionals. We sent a reminder email 3 to 6 months later if we had not received a response.
We entered the collected information in a database and analysed it with the statistical software SPSS Statistics version 25.0 (IBM®, Chicago, IL, USA).
ResultsWe received 97 responses from 69 neonatal units that represented 15 of the 17 autonomous communities in Spain (with the exception of the communities of Extremadura and La Rioja and the autonomous cities of Ceuta and Melilla). Of the 97 responses, 35 (36.1%) were submitted by nursing staff and 62 (63.9%) by medical staff. We received responses from both nurses and doctors for 27 of the participating facilities.
Of the units that submitted a response, 87% were level III units (15 level IIIA, 37 level IIIB, 8 level IIIC) and the remaining 13% were level II units (5 level IIA, 4 level IIB).
A total of 68 units (98.5%) had HFNC, and all used a humidified and heated gas delivery system.
Ninety percent of units used the Fisher & Paykel® Optiflow® nasal cannula to deliver high-flow oxygen therapy, which most cases as the sole system available in the unit (78%). Five percent of the units that used the Fisher & Paykel® cannula used the AIRVO® humidifier and flow generator. In our survey, this system was followed in frequency by the Vapotherm® system (18.6%).
Of all units, 88.7% reported having interfaces in sizes for preterm (PT) newborns, term newborns and infants. Fifty-four percent used HFNC in patients of any gestational age (GA) and weight, while 10% only used it in term newborns. In the subset of units that managed newborns of any GA (levels IIIB and IIIC), 64.4% used HFNC in newborns of any GA or weight, and 4.4% only in term newborns.
Bronchiolitis was the most frequent indication for HFNC in level II units and the second most frequent in the overall sample. Excluding bronchiolitis, the most frequent indication in level III units was weaning from NIV as an alternative to CPAP, followed by respiratory distress in term newborns and post-extubation respiratory support (Table 2).
Indications for the use of high-flow nasal cannulae (percentage of units that used them for each indication, overall and by level of care).
| Indication | Overall | Level II units | Level III units | P |
|---|---|---|---|---|
| NIV weaning | 79.4% | 27.3% | 86% | <.001 |
| Post extubation | 50.5% | 18.2% | 54.7% | .023 |
| Respiratory distress in TNB | 58.8% | 36.4% | 61.6% | .191 |
| Respiratory distress in PTNB | 35.1% | 18.2% | 37.2% | .319 |
| Apnoea of prematurity | 42.3% | 9.1% | 46.5% | .022 |
| Bronchiolitis | 69.1% | 63.6% | 69.8% | .734 |
NIV, non-invasive ventilation; PTNB, preterm newborn; TNB, term newborn.
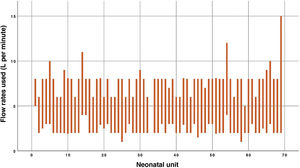
Most respondents used a minimum flow rate of 2 L/min (44/69) and a maximum flow rate was 8 L/min (41/69). Three units used flow rates greater than 10 L/min (Fig. 1).
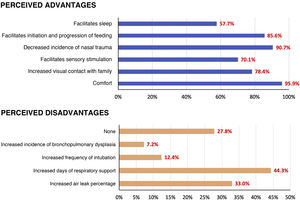
Adverse effects associated with the use of HFNC were reported by 22.7% of respondents: 12.4% reported cases of nasal trauma and 9.3% air leaks. Other described adverse events were airway obstruction, skin ulceration due to the fixation system and hyperthermia (Fig. 2).
In the submitted responses, the perceived benefits exceeded the perceived drawbacks.
Of all respondents, 51.5% reported that their hospital did not have an established HFNC protocol for neonatal patients. All respondents from units that used HFNC (68/69) agreed that nationwide guidelines would be useful and applicable to their neonatal care unit.
DiscussionOur survey on the use of HFNC in neonatal care units in Spain is, to our knowledge, one of the largest conducted to date.
We sent a total of 230 questionnaires to 120 neonatal units and received 97 responses (42% response rate) corresponding to 69 neonatal units (60% of units invited to participate).
The methods used for contact with participants and questionnaire submission were similar to those described in other surveys, like the one conducted in the United Kingdom in 2012,4 in which 170 neonatal units were contacted and 81 responded (48% overall participation rate, which increased to 77% in the subset of level II and III units).
Although the overall response rate was different compared to other surveys and was less than 50%, the sample included units from 15 of the 17 autonomous communities of Spain, with participation of 69 distinct neonatal care units (87% level III).
In Spain, every unit that participated in the survey used HFNC, except for 1 in which its introduction was pending at the time of the study. This differed from the findings of similar surveys conducted in the past.
Thus, a study conducted in the United Kingdom reported its use in 77% of units in 2012,4 another in Canada its use in 89% of units in 2015,9 and one in Japan, also from 2015, its use in only 58% of surveyed neonatal intensive care units (NICUs).6 Our findings reflect the progressive increase in the use of HFNC that has taken place in the past few years.
Practically all units used Fisher & Paykel® and, less frequently, Vapotherm® devices for HFNC therapy. More than half used it in newborns of any GA and weight, a proportion that rose to nearly two thirds in level IIIB and IIIC units.
When it came to the patients that received HFNC therapy, our findings are consistent with those of Mukerji et al9 and Ojha et al.4 In the survey conducted in Canada by Mukerji et al,9 64% of level III units reported having no GA limit for its use (they did not specify the devices used). In the United Kingdom (in a survey of level II and III units),4 71% used HFNC in patients of any GA and 65% in patients with any birth weight, and most units used Vapotherm® (47%) or Fisher & Paykel® (38%) devices. In contrast, in the survey conducted in Japan,6 of the 80 level III neonatal units that participated, only 12% used HFNC in PT infants born before 28 weeks of gestation, and 100% of units used the Fisher & Paykel® system.
However, current expert consensus recommendations and guidelines emphasise GA as a limiting factor in the indication of high-flow oxygen therapy, as the evidence only supports its use post extubation in infants born at or after 28 weeks of gestation. The recommendations for HFNC as initial respiratory support are limiting its use to infants born at or after 30 weeks of gestation with oxygen requirements of less than 30%.16 Even when for the purpose of weaning from CPAP, both GA and weight are factors that affect the success of therapy. It is worth noting that only one survey6 addressed GA as a limiting factor in the indication for nasal high-flow therapy.17
An important aspect to take into account is that one of the safety measures accepted by practically every publication is that the cannula must occupy less than 50% of the nostril diameter to prevent occlusion, which could cause the development of excessive nasopharyngeal pressure.18 Given this safeguard, safe equipment may not always be available, especially in the case of very low birth weight preterm infants.
Bronchiolitis was the most frequent indication in level II units and the second most frequent overall, even though high-flow oxygen therapy has not been found superior to oxygen therapy with conventional cannulae and CPAP in patients with severe bronchiolitis.19
Excluding bronchiolitis, since it is not a disease exclusive of the neonatal period, the most frequent reason for the use of HFNC in level III units was as an alternative to CPAP in weaning from NIV, followed by respiratory distress in term newborns and post-extubation support, and less frequently as initial respiratory support for respiratory distress in PT newborns and in apnoea of prematurity. In comparison, in level II units its use for weaning from NIV was much less frequent and HFNC is only used sporadically for management of respiratory distress or apnoea in PT newborns.20
These results were consistent with the findings in the United Kingdom, where 77% of neonatal units used HFNC therapy as an alternative to CPAP and 71% for weaning from CPAP (results not stratified by level of care).4 In contrast, in Japan fewer than 10% of the units use HFNC for weaning from CPAP frequently (up to 57% use it frequently or occasionally) and fewer than 10% uses them as an alternative to CPAP.6
Our findings reflect the increased use of HFNC in clinical practice despite the lack of sufficient evidence supporting its use. In fact, when it comes to the main indication (weaning from CPAP, 79.4%), there are only 4 prospective studies in the literature comparing both devices, and differences in the populations under study and the applied flow rates hinder the generalization of their results. The current recommendation is to consider the use of HFNC as an alternative to CPAP in PT infants who are clinically stable and/or at risk of developing nasal trauma.13
A consensus review by experts published in the Journal of Perinatology18 notes that the only indication for which there is unanimous consensus and scientific support is the use of HFNC therapy as post-extubation support in preterm infants delivered at or after 28 weeks of gestation. However, at present, it cannot be recommended for management of respiratory distress in extremely PT newborns or for treatment of hyaline membrane disease.20,21
When it comes to the flow rates used in treatment, with the exception of a few isolated units that used higher flows, we found considerable uniformity in both the minimum and the maximum flow rates (2-4 L/min and 6-8 L/min), with nearly 7 out of 10 respondents reporting that the initial flow rate was based on the weight of the newborn.
There is substantial agreement in the literature that the maximum flow rate should not exceed 8 L/min, however, there is more variability in the recommendations regarding the minimal flow rate from which to discontinue support with HFNC, which range from 1 to 4 L/min.3,18
In regard to the perceived advantages and disadvantages of high-flow oxygen, most professionals considered that HFNC improved patient comfort, decreased nasal trauma and facilitated initiation and progression of enteral feeding. The satisfaction of health care professionals in Spain was higher compared to the satisfaction expressed in surveys in Japan (63% considered it decreased the incidence of nasal trauma and 59% that it facilitated feeding)6 and the United Kingdom (46% considered it improved feeding).4
As for the drawbacks, the concerns most frequently expressed by professionals were the risk of bronchopulmonary dysplasia (BPD) and of increasing the duration of respiratory support, although the available evidence in support of the safety of high-flow oxygen does not report differences in the incidence of BPD or the duration of respiratory support compared to weaning with different modalities, such as CPAP or NIV.18
Another frequent concern was the risk of air leak (expressed by 33% of respondents) due to not knowing the PEEP that is being administered. However, fewer than 10% of respondents reported having observed this complication in association with the use of HFNC. A systematic review of the literature did not find a higher incidence of adverse events compared to the use of CPAP.22
As regards nasal trauma, few units in our survey had observed this complication. Nasal trauma associated with the use of CPAP develops in 20% to 100% of patients born before 30 weeks of GA, depending on the series. A meta-analysis conducted in 2018 found that the use of HFNC was associated with a significant reduction in the incidence of nasal trauma compared to CPAP.23
Lastly, when it came to the perceived advantages of HFNC in the context of the current shift towards involving families in infant care, surveyed professionals expressed that the use of HFNC can promote wellbeing in the infant and bonding and physical contact between the infant and the family. More than half of respondents expressed that they felt that it made it easier for patients to sleep and feed by sucking, facilitated sensory stimulation, improved patient comfort and improved visual contact with the family.
Our survey found the absence of established guidelines in more than 50% of participating units (compared to other NIV modalities), a proportion similar to the one found in the survey in the United Kingdom (50%)4 and greater than the one found in Japan,6 where only 4% of the units had an established protocol.
Based on the obtained data, and although the low response rate, the long period allocated for questionnaire submission and the potential impact of the subjectivity of the respondents pose limitations to the interpretation of the findings, it seems reasonable to conclude that the increasingly widespread use of high-flow oxygen therapy is not always consistent with the current evidence on the subject. The lack of protocols at the local and national level, and even the lack of international consensus, result in considerable variability in the use of this modality between sites.
ConclusionHigh-flow oxygen therapy has become a widely used non-invasive respiratory support modality in neonatal units in Spain, despite contradictory evidence from expert reviews and scientific studies.
We found variability in its application between units, although the target population and indications were similar, with differences based on the level of care. Our findings are similar to those of other surveys conducted internationally.
Most units do not have protocols standardising the use of HFNC.
The development of clinical practice guidelines would not only improve everyday clinical practice, but also allow the performance of multicentre studies with large samples to increase our knowledge and gather evidence on the potential indications and benefits of high-flow oxygen therapy in newborns.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Losada OR, Ramón AM, Fernández AG, España VF, Turpin AG, Gómez JJC, et al. Utilización de las cánulas nasales de alto flujo en las unidades neonatales españolas. An Pediatr (Barc). 2022;96:319–325.
Previous presentation: This study was presented as an oral communication at the XXVII Congress of Neonatology and Perinatal Medicine, October 2019, Madrid, Spain.