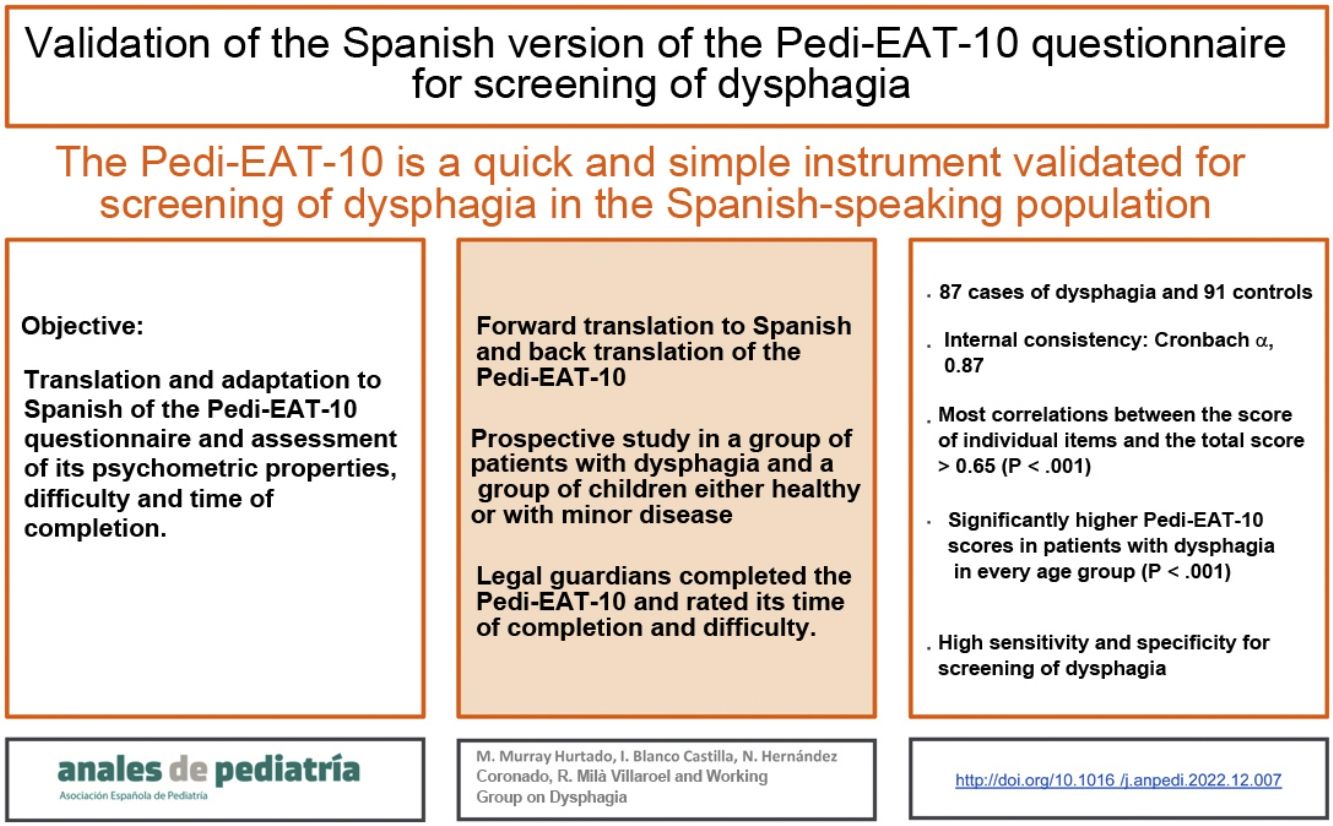
The Pedi-EAT-10 is a quick and simple validated tool for screening for dysphagia in the paediatric age group. The objective of our study was to translate and adapt the scale to Spanish and assess its psychometric properties, level of difficulty and speed of completion.
Patients and methodsFollowing the forward and back translation and the approval by the research team of the Spanish version of the Pedi-EAT-10, we carried out a prospective study in a group of patients with dysphagia and a group of children who were healthy or had minor disease. Their legal guardians completed the questionnaire and reported the duration and difficulty of the test.
ResultsThe study included 87 cases of dysphagia and 91 controls. The Cronbach alpha for internal consistency was 0.87. Most correlations between single item scores and the total scale score were greater than 0.65 (P < .001). The Pedi-EAT-10 scores were significantly higher in patients with dysphagia in every age group (P < .001), evincing a high sensibility and specificity for the screening of dysphagia. In the control group, the mean time taken to complete the questionnaire was 2.18 ± 1.98 min, and all participants found it easy.
ConclusionsWe verified the validity, reliability and internal consistency of the Spanish version of the Pedi-EAT-10. It is an easy and quick instrument that can be used for screening of dysphagia in paediatric clinical practice.
El Pedi-EAT-10 es un instrumento validado, sencillo y rápido para el despistaje de disfagia en la edad pediátrica. El estudio tiene como objetivo traducir y adaptar al español la escala y comprobar sus propiedades psicométricas, dificultad y rapidez de cumplimentación.
Pacientes y métodosTras la traducción, retrotraducción y aprobación por los investigadores de la versión en español del Pedi-EAT-10, se realizó un estudio prospectivo con un grupo de pacientes con disfagia y otro grupo de niños sanos o con patología menor. Sus tutores legales cumplimentaron el test y valoraron la duración y dificultad del test.
ResultadosEl estudio incluyó 87 casos con disfagia y 91 controles. El coeficiente de consistencia interna alfa de Cronbach fue 0,87. La mayoría de correlaciones entre las puntuaciones de cada ítem y el total de la escala fueron > 0,65 (P < ,001). Las puntuaciones del Pedi-EAT-10 fueron significativamente más altas en los pacientes con disfagia en todos los grupos de edad (P < ,001), mostrando así una alta sensibilidad y especificidad para el cribado de disfagia. En el grupo control, el tiempo medio de administración fue de 2,18 ± 1,98 minutos y todos consideraron fácil su cumplimentación.
ConclusionesSe ha comprobado la validez, fiabilidad y consistencia interna de la versión en español del Pedi-EAT-10. Es un instrumento fácil y rápido, útil para el despistaje de la disfagia en la práctica clínica en Pediatría.
Dysphagia is a symptom caused by a structural or functional abnormality in the oropharynx or oesophagus, and may affect the effectiveness or safety of swallowing, resulting in undernutrition, dehydration or bronchial aspiration, among other possible complications that increase morbidity and mortality, have a negative impact on the quality of life of patients and their families and generate high health care costs.1,2
In the paediatric age group, swallowing problems can develop as a manifestation of many diseases, and their incidence and prevalence are underestimated (up to 20% of children with normal development, 80% of children with developmental disorders and 85% of medically complex children experience feeding difficulties of a diverse nature).3 The incidence of oropharyngeal dysphagia in the paediatric population is estimated at 1%, with higher estimates for certain risk groups.4 In many cases, it resolves spontaneously, but it becomes chronic in 3%–10% of cases.5
Several screening tools have been developed to help identify early and easily patients with characteristics suggestive of dysphagia that require evaluation by a specialist.6,7
Several screening questionnaires have been developed for adults,8–10 but due to their limitations in terms of complexity, length and duration, none has been routinely applied in clinical practice. The Eating Assessment Tool-10 (EAT-10) is an analogical, verbal, unidimensional self-report measure that takes little time to complete8 validated in adults and that has proven useful to assess of the severity of symptoms, predict the risk of aspiration in patients with dysphagia and monitor treatment. A Spanish version of this tool has also been validated.6
When it comes to the paediatric population, several instruments are available to assess swallowing and feeding, although with substantial variability in terms of their design, assessed variables, indications and target population, and there are few data on their quality, validity and reliability.11,12 The Pediatric Eating Assessment Tool (PediEAT) is a parent-report questionnaire comprising 78 items developed to identify “problematic eating behaviours” in children aged 6 months to 7 years.13,14 However, none of these instruments have been developed specifically for the detection of dysphagia and many are too laborious for routine use.
The Pedi-EAT-10 screening tool, adapted from the equivalent tool for adults (EAT-10), is the first validated instrument specifically designed for the identification of children at high risk of aspiration. It is a simple parent-proxy self-report scale, consisting of 10 items, that has been found to be a practical, easy to administer and inexpensive tool to predict the risk of aspiration in paediatric patients aged 18 months to 18 years.15 A total score of 4 or more points is considered abnormal, and a total score of 13 points or higher has exhibited a high sensitivity and specificity in the prediction of penetration and/or aspiration.16 Thus, it can be used as a tool with discriminatory power to detect the risk of dysphagia and aspiration in children, with higher scores indicative of greater risk.16 Although it was originally tested in children with cerebral palsy, it has been applied to different diseases13,16,17 and validated in other languages,18 although it has not been systematically validated for use in the Spanish population.
The aim of our study was to translate to Spanish and culturally adapt the Pedi-EAT-10 and assess its psychometric properties and ease and time of completion.
Material and methodsStudy designWe conducted an observational case-control study to validate the Spanish version of the Pedi-EAT-10 and assess its psychometric properties and internal consistency as a diagnostic tool.
Ethical considerationsThe study was conducted in adherence to the most recent revision of the Declaration of Helsinki and current law and regulations in Europe and Spain. We obtained the informed consent of the legal guardians of the participants through the signing of the corresponding form.
The study was approved by the Ethics Committee of the hospital (code CHUC_2021_128).
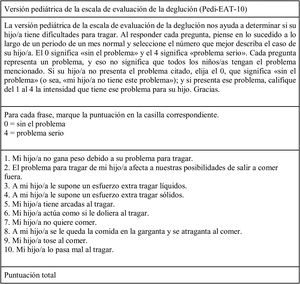
Description of the instrumentThe Pedi-EAT-10 is an analogical verbal self-report scale that yields a direct score to assess specific symptoms of dysphagia. The validity and reliability of the original scale (in English) have been demonstrated and reported in a previous publication.15 It is a 10-item questionnaire developed by a multidisciplinary team of experts in swallowing disorders. To obtain the score, the parent must provide a subjective rating on a scale from 0 to 4 points, where 0 indicates absence of the problem and 4 presence of the problem in its severest form. The final score is obtained by adding the 10 item scores. A final score of 4 points or greater is indicative of abnormal swallowing.
Translation to SpanishThe validation process of the Spanish version of the Pedi-EAT-10 started with the translation to Spanish of the original version published in English followed by back translation to English by 2 independent teams of bilingual translators. This version was revised by experts in nutrition and speech therapy to obtain and adapted version better fitting the clinical context in Spain. When it had been revised and approved, it was once again back translated to English, and the translation was found to be faithful to the original. The resulting final text can be found in Fig. 1. We obtained permission to translate and adapt the questionnaire from the lead author of the original version (SS Arslan).
Study sampleWe recruited 91 participants of both sexes aged 6 months to 18 years with a diagnosis of dysphagia cases) and 91 healthy individuals (controls). The necessary sample size was calculated for a power of 80% (with an alpha risk of 5% and a beta risk of 20%) in detecting a statistically significant differences of 9 points in the total score of the test Pedi-EAT-10 between the patients without dysphagia (controls) and the patients with a diagnosis of dysphagia (cases) in a bilateral test.
Participants with dysphagia (case group) were recruited prospectively and consecutively among the children referred to the paediatric nutrition clinic with a history suggestive of oropharyngeal dysphagia, usually with some form of predisposing underlying disease. the diagnosis of dysphagia was confirmed in all of them following the full evaluation by the paediatrician specialised in nutrition and the speech therapist, who carried out an exhaustive assessment including the completion of the Pedi-EAT-10, a focused history taking, questionnaires to assess sialorrhoea and dental hygiene, direct observation of prescribed intake and a volume-viscosity swallow test (V-VST). In addition, 24% underwent a videofluoroscopic swallowing study (VFSS) and 39% a fibreoptic endoscopic evaluation of swallowing (FEES), per the judgment of the clinician.
The rest of patients (control group) were recruited prospectively among children who were healthy or had minor disease that would not manifest with or contribute to dysphagia and with no manifestations compatible with dysphagia from the primary care caseloads of the North Tenerife health care area. Controls were matched for age and sex with cases and recruited in the initial phase.
ProtocolTo assess the construct validity of the Pedi-EAT-10, the questionnaire was completed by the parents or primary caregivers of the child under the supervision of a clinical provider (speech therapist/paediatrician). To pursue homogeneity in the answers supervising providers would give to the questions that participants may bring up during the completion of the questionnaire, precise instructions were drafted and provided to all supervising providers. In addition to providing the data collected in the screening tool (Pedi-EAT-10), participants also filled out an ad hoc questionnaire created to collect basic information such as the age and sex of the child, underlying disease or chronic treatments and information on the time it took to complete the Pedi-EAT-10 and the perceived difficulty in completing it. In the case group, we also recorded the primary diagnosis with the corresponding International Classification of Diseases 11th Revision (ICD-11) code.
Statistical analysisAll the analyses were made based on the available data, without imputation of missing data, and describing the number of values missing in each analysis. For all tests performed, the level of statistical significance was 5% (α = 0.05). The statistical analysis was performed with the software IBM SPSS version 25.0 (IBM Corp, Armonk, NY, USA).
We conducted a descriptive analysis of all the data entered in the data collection form. Tests were used to assess whether quantitative data followed a normal distribution (Kolmogorov and Shapiro-Wilks) and established that this was the case for all study variables under study. To describe quantitative variables, we used the mean and standard deviation. For categorical variables, we used absolute frequencies and percentages.
To assess whether there were significant differences between the two groups in the scores for each item and the total, we compared the means (Student t-test for independent samples), and to determine whether the age group was significantly associated with the scores, we conducted an analysis of variance (ANOVA) adjusted for multiple comparisons with the Bonferroni correction. To assess whether there were significant differences between group in qualitative variables, we used a test to compare independent proportions (χ2). We assessed the internal consistency of the instrument by means of the Cronbach alpha. We used the Pearson correlation coefficient to measure the correlation between each individual item and the total score. We assessed the sensibility and specificity by means of receiver-operating characteristic (ROC) curves.
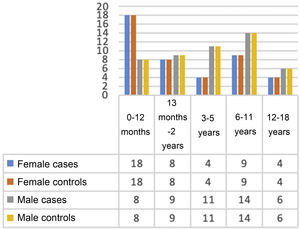
ResultsSociodemographic and clinical characteristics of the patientsThe study included a total of 182 participants (91 controls and 91 cases with dysphagia), divided by age group to match participants and obtain comparable groups. However, the Pedi-EAT-10 forms for 4 children with dysphagia were misplaced during the study period, so these 4 cases were excluded from the analysis.
There were no significant differences in age or sex between participants in the two groups, as cases and controls had been matched (Fig. 2).
In the control group, 18.7% had a controlled underlying disease that did not cause dysphagia a priori.
In the cage group, there was a majority of children with neurologic disease (46.2%, most frequently genetic encephalopathy and disease of unknown aetiology). Other causes of dysphagia were prematurity (7%), pulmonary disease (7%) or gastrointestinal disease (6%). Up to 15.4% of children in the case group had a combination of diseases affecting different systems and 6 patients had no known comorbidities (6%).
Adequacy of the Pedi-EAT-10 to SpanishThe questionnaire was easy to complete and took little time: in the dysphagia case group, 100% of parents completed it in full, leaving no items unanswered and exhibiting no reluctance to provide answers.
In the control group, we were able to assess more objectively the perceived difficulty: 82.4% rated the Pedi-EAT-10 as very easy and 17.6% as easy, and no participant rated it as being moderately difficult, difficult or very difficult. The mean time devoted to completing the questionnaire was 2.18 min (from 1 to 10 min). Thus, we could corroborate that this instrument can also be completed in little time in the Spanish version.
The researchers found no salient biases: tendency to avoid extreme responses, to answer what is perceived as socially acceptable, to choose the same answer in successive questions etc.
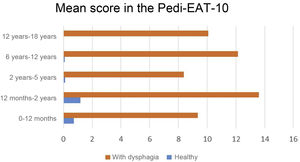
Validation of the Spanish version of the Pedi-EAT-10In the group of 87 children with dysphagia, 73 had positive results indicative of dysphagia (total score ≥ 4 points), while in the group of 91 healthy controls, only 5 (all aged 6–24 months and without diseases or treatments that could explain the results or findings suggestive of dysphagia in the focused history taking). In every age group, the total score of the questionnaire was significantly different between children with dysphagia and controls (P < .001) (Fig. 3).
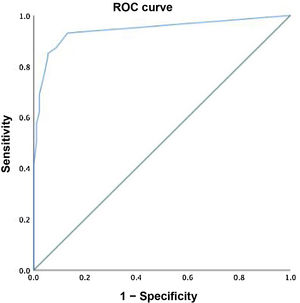
Using these data, we found a high sensitivity and specificity, of 83.9% and 94.5%, respectively, with a positive predictive value of 86% and a negative predictive value of 93.6%. This was reflected in the plotted ROC curve, with an excellent area under the curve of 0.943 (Fig. 4).
In the assessment of the reliability of the instrument and its internal consistency, we obtained a Cronbach α of 0.865 (since it was within the ideal value range, 0.70−0.90, we ruled out poor consistency, redundancy and duplication). Also, for each of the 10 items, we calculated the Cronbach α that would result if that item were removed, and found values greater than 0.8 in every case (Table 1). This suggests that even if one of the items is left unanswered, the reliability and consistency of the test continue to be high.
Cronbach α obtained with the removal of individual items and correlation between the total score in the Pedi-EAT-10 and individual item scores.
| Cronbach α obtained removing the item | Correlation between the total score of the Pedi-EAT-10 and each item | |
|---|---|---|
| Item 1 (Mi hijo/a no gana peso debido a su problema para tragar) | 0.851 | 0.682 |
| Item 2 (El problema para tragar de mi hijo/a afecta a nuestras posibilidades de salir a comer fuera) | 0.843 | 0.769 |
| Item 3 (A mi hijo/a le supone un esfuerzo extra tragar líquidos) | 0.858 | 0.643 |
| Item 4 (A mi hijo/a le supone un esfuerzo extra tragar sólidos) | 0.852 | 0.733 |
| Item 5 (Mi hijo/a tiene arcadas al tragar) | 0.849 | 0.699 |
| Item 6 (Mi hijo/a actúa como si le doliera al tragar) | 0.864 | 0.597 |
| Item 7 (Mi hijo/a no quiere comer) | 0.853 | 0.657 |
| Item 8 (A mi hijo/a se le queda la comida en la garganta y se atraganta al comer) | 0.852 | 0.669 |
| Item 9 (Mi hijo/a tose al comer) | 0.846 | 0.733 |
| Item 10 (Mi hijo/a lo pasa mal al tragar) | 0.852 | 0.674 |
Most of the correlations of individual item scores with the total score for the Pedi-EAT-10 were greater than 0.65, which indicates a good correlation between the total score and the answers given to each of the items, all with a P value of less than .001 (Table 1). This confirmed the adequacy of the items that comprise the Pedi-EAT-10.
We analysed each of the items of the test by age group. The different in the score for each individual item comparing the case and control groups was statistically significant in every age group (P < .001).
DiscussionThe results confirmed the validity of the Spanish version of the Pedi-EAT-10, in addition to its internal consistency and reliability, while evincing a high sensitivity and specificity in the population under study, consistent with the original version, so it is reasonable to state that this is a useful screening tool for dysphagia in the Spanish-speaking paediatric population.
Most of the parents of the participants considered the instrument very simple, it was completed in a few minutes and we did not get any blank responses, so, as was the case with the original version, the Spanish questionnaire was comprehensible and had an adequate length, and could be self-completed, which makes supervision by qualified staff unnecessary except to provide clarification in particular instances, so that it is feasible in everyday clinical practice.
At present, there are no other screening tools for dysphagia in Spanish for the paediatric population, and few are available in other languages,11,19 so the validation of the Pedi-EAT-10 is a significant contribution for primary care, specialty care or speech therapy clinics, among others, in the Spanish-speaking population.
We ought to mention some of the caveats of the original version of the Pedi-EAT-10, which will obviously remain in the Spanish version, as it has just only been translated and validated: the fact that it was adapted from an instrument designed for adults (EAT-10), that it was initially only validated in children with cerebral palsy compared to controls, overlooking other causes of dysphagia, and that children aged less than 18 months were not included (in fact, this is the age group in which we found healthy children without dysphagia with total scores that, while low, could generate some uncertainty, so that results should be interpreted with caution in very young children). At any rate, as is the case of any other screening tool, the results must be corroborated with an appropriate clinical evaluation.
We ought to mention that while we were conducting this study, we became aware of a master thesis project that pursued the same objective and which yielded conclusions similar to ours. However, the sample size in this thesis was probably insufficient to achieve an adequate statistical power. Furthermore, our analysis evinced the sensitivity, specificity, reliability and internal consistency of the test, aspects that were not explored in the other project.20
The main limitations of our study are that we did not make a precise assessment of the difficulty perceived by caregivers of children with dysphagia, nor recorded the exact time it took them to complete the test, aspects that were documented by the speech therapist based on her subjective appraisal, compared to the control group, in which they were actually recorded. Furthermore, the presence of disease in some of the controls, independently of its kind, could bring up reasonable doubts regarding possible dysphagia or produce confounding symptoms, although none of these patients had any such suggestive features per the judgment of the paediatrician, nor did their parents assign more points in the scale compared to the parents of healthy controls. Lastly, the age range in our sample was broader compared to the age range in which the original version was studied, so our findings for children aged less than 18 months may need to be interpreted with caution. Previous studies have shown that age has a significant impact on feeding behaviour in healthy children,5 so it would be interesting to explore these aspects in future studies using the Spanish version of the Pedi-EAT-10.
As for the strengths of the study, we ought to mention the sample size, which was very similar to the one used in the development of the original version of the Pedi-EAT-10 and sufficient to achieve an adequate statistical power, and having included a broad range of ages and underlying diseases that cause dysphagia in the case group, upon which we found significant differences in the scores compared to controls in every age group.
With regard to future research, it would be interesting to carry out a test-retest analysis to confirm our results in the study population and corroborate the usefulness of the Spanish version of the Pedi-EAT-10 in everyday clinical practice.
ConclusionsOur results confirmed the psychometric properties, reliability, internal consistency and validity of the Spanish version of the Pedi-EAT-10, demonstrating anew that this is a useful tool for the screening of dysphagia in the paediatric population.
Therefore, this version of the Pedi-EAT-10, faithful to the original, offers a reliable screening tool that can differentiate patients with relevant features of dysphagia and risk of bronchial aspiration who require a more throughout and specialised assessment.
FundingThis study received funding from Fresenius Kabi España S.A.U. through the Fundación Instituto de Investigación Sanitaria de Canarias (FIISC) under project code D21/006/. Fresenius Kabi España S.A.U. contributed to the hiring of the two translator teams, funded the grant for the collaboration of the speech therapist Noemi Hernández in the research project and paid the fees of Raimon Milá for the statistical analysis. Beyond this, the corporation was not involved in the design of the study, the data collection, analysis and interpretation, the drafting of the article or any decisions regarding its publication.
Conflicts of interestMercedes Murray has received financial aid in the past 5 years to cover travel, lodging and registration expenses to attend courses and congresses as well as fees for her participation as an instructor in workshops and conferences from Nestlé Health Science, Abbott, Nutricia, Fresenius Kabi, Mead & Johnson, Casen Recordati and Lactalis, although she denies any conflicting interests that could interfere with the results of this study. Noemi Hernández Coronado and Raimon Milà Villaroel receive fees from Fresenius Kabi S.A.U, but declare that this economic relationship does not entail a conflict of interest that would affect the results of the study. Irene Blanco Castilla and the authors members of the Working Group on Dysphagia have no conflicts of interest to declare in relation to this study.
Díaz-Flores Varelae, Desiré González Gonzálezf, Juan AntonioHernández Poncee, Marta López Garcíag, Gema Perera de Leónh, Cristina Quintana Herrerai, Tania Rodríguez Méndeze, María Cecilia Salom Lucenaj, Ángela Seoane Ceak, María Eloisa Suárez Hernándezl and EduardoValerio Hernándezm
e Department of Diagnostic Imaging. Complejo Hospitalario Universitario de Canarias. La Laguna, Santa Cruz de Tenerife.
f Paediatrician. Centro de Salud La Vera. Primary Care Administration of Tenerife.
g Paediatrician. Centro de Salud Icod de los Vinos. Primary Care Administration of Tenerife.
h Paediatrician. Centro de Salud Tegueste. Primary Care Administration of Tenerife.
i Paediatrician. Centro de Salud Tacoronte. Primary Care Administration of Tenerife.
j Department of Otorhinolaryngology. Complejo Hospitalario Universitario de Canarias. La Laguna, Santa Cruz de Tenerife.
k Paediatrician. Centro de Salud San Benito, La Laguna. Primary Care Administration of Tenerife.
l Paediatrician. Centro de Salud Tejina-Tegueste. Primary Care Administration of Tenerife.
m Paediatrician. Centro de Salud Casco-Botánico. Primary Care Administration of Tenerife.