To assess the effects of early or late clamping of the umbilical cord in at-term newborns, assessing the levels of hemoglobin, hematocrit, and ferritin, and their correlation with some of the complications.
Patients and methodsA prospective study of healthy newborns at term or born by dystotic or eutocic delivery in our hospital between May 2009 and May 2010. Patients were assigned according to the time of clamping, group 1 (<60s), group 2 (1 to <2min), and group 3 (2–3min). Laboratory tests were performed at birth and at 48h of life, assessing the levels of hemoglobin, hematocrit, ferritin, and bilirubin. The risk of polycythemia, respiratory distress syndrome, neonatal phototherapy or admission to the Intensive Care Unit and the hospital stay, were evaluated.
ResultsA total of 242 patients were included: group 1 (g1=80), group 2 (g2=31) and group 3 (g3=131). The background maternal and neonatal characteristics were similar in all sets. The first test showed significant differences in ferritin levels in those infants with delayed clamping (g1: 111mg/dl, g2: 125mg/dl, g3: 173mg/dl; p<0.01). In the second analysis the values of hemoglobin (g1: 17.3g/dl, g2: 18.9g/dl, g3: 19.2g/dl; p<0.01), hematocrit (g1: 53.4%, g2: 58%, g3: 59%; p<0.01) and ferritin (g1: 254mg/dl, g2: 254.7mg/dl, g3: 313mg/dl; p=0.008) were statistically higher in this group. As regards complications, a significant increase was observed in the number of cases of polycythemia symptoms in group 3.
ConclusionsThe late cord clamping is associated with an increase in hematocrit, hemoglobin and ferritin at 48h of life, as well as an increased risk of polycythemia present with symptoms.
Evaluar los efectos del pinzamiento precoz o tardío del cordón umbilical en recién nacidos a término y su correlación con los niveles de hemoglobina, hematocrito, ferritina y ciertas complicaciones neonatales.
Pacientes y métodosEstudio prospectivo en recién nacidos sanos, a término, nacidos por parto eutócico o distócico en nuestro hospital, entre mayo del 2009 y mayo del 2010. Se asignó a los pacientes según el tiempo de pinzamiento: grupo 1 (<60 s), grupo 2 (1 a <2 min) y grupo 3 (2 a 3 min). Se realizaron análisis al momento del nacimiento y a las 48 h de vida, valorando los niveles de hemoglobina, hematocrito, ferritina y bilirrubina. Se evalúo el riesgo de aparición de policitemia, síndrome distrés respiratorio, fototerapia o ingreso en la Unidad de Cuidados Intensivos neonatal y el tiempo de estancia hospitalaria.
ResultadosSe incluyó a 242 pacientes: grupo 1 (g1=80), grupo 2 (g2=31) y grupo 3 (g3=131). Los antecedentes maternos y las características neonatales fueron similares en todas las categorías. El primer análisis demostró diferencias significativas en los niveles de ferritina de aquellos recién nacidos con pinzamiento más tardío (g1: 111 mg/dl, g2: 125 mg/dl, g3: 173 mg/dl; p<0,01). En el segundo análisis los valores de hemoglobina (g1: 17,3 g/dl, g2: 18,9 g/dl, g3: 19,2 g/dl; p<0,01), hematocrito (g1: 53,4%, g2: 58%, g3: 59%; p<0,01) y ferritina (g1: 254 mg/dl, g2: 254,7 mg/dl, g3: 313 mg/dl; p=0,008), fueron estadísticamente mayores en este mismo grupo. Al evaluar las complicaciones, observamos un aumento significativo en el número de casos de policitemia asintomática en el grupo 3.
ConclusionesEl pinzamiento tardío del cordón umbilical se asocia a un aumento en los niveles de hemoglobina, hematocrito y ferritina a las 48 h de vida y en el número de casos de policitemia asintomática.
Until recently, clamping of the umbilical cord was done a few seconds after birth. This was based on the rationale that early interruption of foetal circulation would be beneficial to the newborn by helping prevent complications such as polycythaemia, hyperviscosity, hyperbilirubinaemia, or transient tachypnoea.1,2 Likewise, early clamping has been recommended for certain situations, such as multiple births, to prevent twin-to-twin transfusion syndrome, and in children of HIV-positive mothers to minimise the risk of transmission.2,3
At present, there is a tendency to recommend increasingly delayed clamping of the cord, on average 2–3min after birth, and in some cases until the cord has ceased pulsating, after delivery.4,5 This shift is due to the multiple benefits that this practise can afford: higher haemoglobin and haematocrit levels, increased iron stores, and earlier and longer mother–child contact.6–9 It has been described that in preterm babies this practise also reduces the risk of intraventricular haemorrhage.10–13
Under normal conditions, the blood volume of the foetus is around 70ml/kg and the placenta contains about 45ml/kg of foetal blood. When clamping of the cord is delayed, approximately 20–35ml/kg of blood can be transfused to the newborn, increasing the foetal blood volume by up to 50%.14 This additional supply of red blood cells would also increase the iron reserves of the neonate by 30–50mg, reducing the risk of iron-deficiency anaemia in the first year of life. 15,16
Despite the published data, there is no global consensus on the ideal clamping time, and even on a European level, the health protocols and policies across different countries have not reached an agreement on this issue.17 However, a trend is starting to materialise in the current recommendations for neonatal resuscitation, which stipulate a delay in cord clamping of at least one minute for uncompromised babies.18,19
Lastly, this debate has led to an increasing number of studies20–27 focused on determining the optimal timing for shutting down foeto-placental circulation and the advantages and/or disadvantages of delaying clamping of the umbilical cord.
ObjectiveThe aim of this study was to assess the effects of early clamping (<60s) and delayed clamping (between 1–2 and 2–3min) in at-term neonates born at the Fundació Hospital Sant Jaume d’Olot, evaluating potential differences in haemoglobin, haematocrit, and ferritin levels at 48h of age, and the correlation of these levels with neonatal complications such as polycythaemia, hyperbilirubinaemia, respiratory distress syndrome, or admission to the neonatal intensive care unit.
Materials and methodsPopulation and design of the studyWe conducted a prospective study that included all healthy neonates born at term (between 37 and 42 weeks) in normal and complicated (instrumental or caesarean) deliveries in the paediatrics department of our institution (Fundació Hospital Sant Jaume d’Olot, Level I), in the period between May 2009 and May 2010.
Exclusion criteriaNeonates that met any of the following conditions were excluded from the study: born in a multiple delivery; born to a mother that had gestational diabetes, eclampsia, pre-eclampsia, HIV infection, or a positive indirect Coombs test result; mild or severe neonatal asphyxia; congenital malformations; and congenital heart disease.
Assignment into groupsOnce a candidate for inclusion was identified, the on-call midwife informed the parents of the objectives and methods of the ongoing study. If the parents decided to authorise the participation of the neonate, they were asked to sign an informed consent form.
Patients were assigned to groups according to the timing of the interruption of blood flow between the placenta and the neonate: early clamping (<60s), intermediate clamping (between 1 and 2min), and late clamping (between 2 and 3min). The appropriate time to do the clamping was determined by the midwife based on her experience, clinical criteria, and the condition of the newborn.
Process and variablesTo collect information, we created a standardised form divided in 3 sections. The first section was filled out by the midwife and included the mother's personal data, data on the haemoglobin and ferritin levels of the third trimester of the pregnancy, iron supplementation during pregnancy, and the presence or absence of tobacco use. This first section also contained data on the type of delivery, the weight, length, and head circumference of the neonate at birth, the Apgar score, gestational age, and timing of the clamping of the cord.
A 1-ml blood sample was drawn from the cord of each neonate and tested in the laboratory of our centre to measure the levels of haemoglobin, haematocrit, and ferritin. The test results were entered in the second section of the form by the on-call paediatrician, along with other data pertaining to the newborn, such as the name, medical record number, sex, and a code for identifying the patient in the database. The newborns remained hospitalised for a period of 2–4 days, during which they received a routine physical check-up by the staff paediatrician.
At 48h of age, when venous blood is drawn to screen for congenital metabolic disorders, we measured the levels of haemoglobin, haematocrit, ferritin, and bilirubin for the second time. The results were added to the patient's form, along with the current weight of the neonate and the percentage of birth weight lost.
The third section of the form documented the presence or absence of polycythaemia (defined as haemoglobin >20g/dl and/or haematocrit >65%), respiratory distress syndrome, jaundice requiring phototherapy, and admission to the neonatal intensive care unit. This section also included the length of the hospital stay in days.
Statistical analysisWe entered the data from the forms in a database built with Microsoft Access®.
We expressed descriptive data as absolute frequencies and percentages for qualitative variables, and as mean±standard deviation and interquartile range for quantitative variables. We used the Shapiro–Wilk test to check the normality of the quantitative variable distributions.
To compare quantitative variables between the 3 groups of the study, we used ANOVA if the variables had a normal distribution, and otherwise used the non-parametric Kruskal–Wallis test.
We used the chi-square test of Fisher's exact test as appropriate to compare qualitative variables.
The statistical significance level was set at p<0.05, and the statistical analysis was performed using SPSS version 12.0.
ResultsA total of 449 live births occurred between May 2009 and May 2010, and 242 of these newborns were included in the study. They were distributed into groups as follows: 80 (33%) in Group 1 (<60s), 31 (12.8%) in Group 2 (1–2min) and 131 (54%) in Group 3 (2–3min).
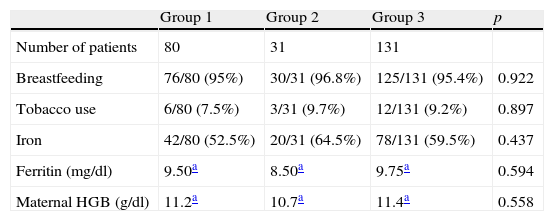
Table 1 contains the main data and characteristics of the mothers. Breastfeeding was the mode of feeding practised by practically all mothers (95.4%). The prevalence of tobacco use during gestation was low, 8.6% of the mothers. Iron supplement intake and ferritin and haemoglobin concentrations in the mother during the third trimester of gestation were similar across the sample. We found no significant differences between the three groups when we analysed these variables.
Maternal data.
| Group 1 | Group 2 | Group 3 | p | |
| Number of patients | 80 | 31 | 131 | |
| Breastfeeding | 76/80 (95%) | 30/31 (96.8%) | 125/131 (95.4%) | 0.922 |
| Tobacco use | 6/80 (7.5%) | 3/31 (9.7%) | 12/131 (9.2%) | 0.897 |
| Iron | 42/80 (52.5%) | 20/31 (64.5%) | 78/131 (59.5%) | 0.437 |
| Ferritin (mg/dl) | 9.50a | 8.50a | 9.75a | 0.594 |
| Maternal HGB (g/dl) | 11.2a | 10.7a | 11.4a | 0.558 |
HGB: haemoglobin.
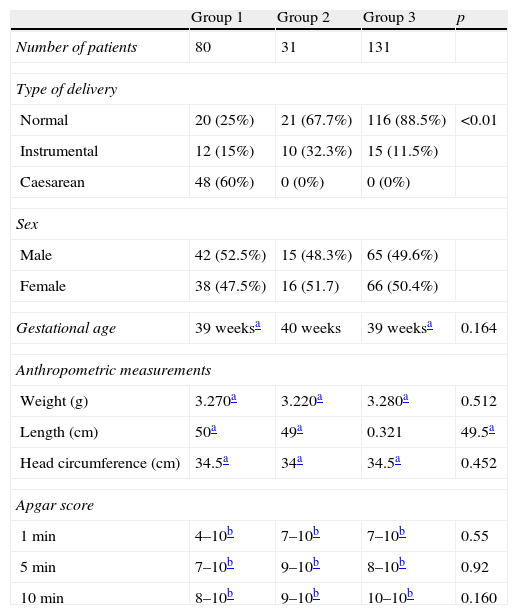
Normal deliveries were most frequent, accounting for 65% of the sample (157 births), followed by caesarean deliveries (48 births, 19.8%) and instrumental deliveries (37 births, 15.2%). The average gestational age was 39 weeks and the sex distribution was 122 boys and 120 girls.
The median weight, length, and head circumference at birth were similar in all groups. The average Apgar score was similar in the 3 groups, but the score's minimum values were lower in the early clamping groups (see Table 2).
Birth data.
| Group 1 | Group 2 | Group 3 | p | |
| Number of patients | 80 | 31 | 131 | |
| Type of delivery | ||||
| Normal | 20 (25%) | 21 (67.7%) | 116 (88.5%) | <0.01 |
| Instrumental | 12 (15%) | 10 (32.3%) | 15 (11.5%) | |
| Caesarean | 48 (60%) | 0 (0%) | 0 (0%) | |
| Sex | ||||
| Male | 42 (52.5%) | 15 (48.3%) | 65 (49.6%) | |
| Female | 38 (47.5%) | 16 (51.7) | 66 (50.4%) | |
| Gestational age | 39 weeksa | 40 weeks | 39 weeksa | 0.164 |
| Anthropometric measurements | ||||
| Weight (g) | 3.270a | 3.220a | 3.280a | 0.512 |
| Length (cm) | 50a | 49a | 0.321 | 49.5a |
| Head circumference (cm) | 34.5a | 34a | 34.5a | 0.452 |
| Apgar score | ||||
| 1min | 4–10b | 7–10b | 7–10b | 0.55 |
| 5min | 7–10b | 9–10b | 8–10b | 0.92 |
| 10min | 8–10b | 9–10b | 10–10b | 0.160 |
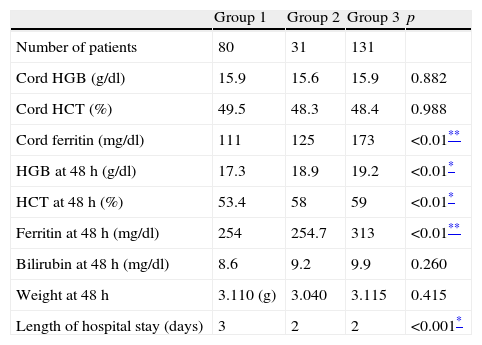
The results of cord blood analysis (Table 3) showed that haemoglobin and haematocrit levels were documented for every participant, and that there were no statistically significant differences between the three groups. On the other hand, cord blood ferritin values were documented in 230 of the 242 participants, and showed a significant difference in the median of Group 3, which was greater than the medians of Group 1 and Group 2.
Blood test results.
| Group 1 | Group 2 | Group 3 | p | |
| Number of patients | 80 | 31 | 131 | |
| Cord HGB (g/dl) | 15.9 | 15.6 | 15.9 | 0.882 |
| Cord HCT (%) | 49.5 | 48.3 | 48.4 | 0.988 |
| Cord ferritin (mg/dl) | 111 | 125 | 173 | <0.01** |
| HGB at 48h (g/dl) | 17.3 | 18.9 | 19.2 | <0.01* |
| HCT at 48h (%) | 53.4 | 58 | 59 | <0.01* |
| Ferritin at 48h (mg/dl) | 254 | 254.7 | 313 | <0.01** |
| Bilirubin at 48h (mg/dl) | 8.6 | 9.2 | 9.9 | 0.260 |
| Weight at 48h | 3.110 (g) | 3.040 | 3.115 | 0.415 |
| Length of hospital stay (days) | 3 | 2 | 2 | <0.001* |
All the values correspond to the median. HCT: haematocrit; HGB: haemoglobin.
The analysis performed at 48h after birth showed higher levels of haemoglobin, haematocrit and ferritin in group 3, and this difference was statistically significant. Data on haemoglobin and haematocrit levels were available for 100% of the sample, and data on ferritin for 91% of the sample.
The bilirubin levels at 48h (documented in 224 of the 242 participants) showed no significant differences between the 3 groups.
The median weight of patients at 48h after birth was similar in all groups. The hospital stay was significantly shorter in patients in Group 2 and Group 3 compared to patients in Group 1 (which included all neonates delivered by caesarean section).
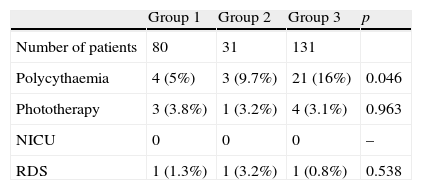
Table 4 shows the statistical analysis of variables pertaining to neonatal complications. We found no significant differences in these variables with the exception of polycythaemia, of which there was a significantly higher percentage of cases in patients from the late clamping group.
DiscussionThe main objective of this prospective study was to assess the correlation between different timings for clamping the cord and certain neonatal complications associated to the volume of blood in the newborn. In this regard, our results only showed a significant difference for polycythaemia. We found no differences in the remaining variables (jaundice requiring phototherapy, respiratory distress syndrome, and admission to the neonatal intensive care unit). Furthermore, when we analysed the cases of polycythaemia, we found that all of them were asymptomatic and none required any treatment or specific care other than doing the test again to see whether the levels had normalised. Based on these results, we can say that clamping the cord between 0 and 3min is a safe practise that does not lead to increased morbidity.
We set arbitrary time intervals for each group (<60s, 1–2min, and 2–3min), as no clear recommendations existed at the beginning of this study. Later on, official guidelines for neonatal resuscitation were published that recommended the cord be clamped after the first 60s of life in healthy newborns. In addition, one of the most recent literature reviews (of 15 studies comprising 3911 infants)22 also defined early clamping as clamping performed within the first 60s of life. For these reasons, we believe that the time intervals we established were appropriate.
Although our study had a small sample and a short duration, both sufficed to obtain statistical results with adequate power. Likewise, the samples are comparable in every aspect under study, with the exception of type of delivery, as we had expected. Complicated deliveries prevailed in Group 1 (early clamping) because this group included all the caesarean deliveries, in which clamping has been and continues to be done within the first minute of life. The analysis of the “length of hospital stay” also showed a statistically significant greater number of days in the early clamping group for the same reason (caesarean sections).
We should also note the difference in the number of newborns included in each group. The late clamping group included over half of the sample. This may be due to the fact that our hospital is accredited for assisting natural deliveries and has a different philosophy that affects how certain decisions are made.
The results of the cord blood analysis showed significantly higher ferritin levels in the late clamping group, which is probably due to the increased volume of placental transfusion. It must be taken into account that there were no significant differences in the mothers’ ferritin levels.
In the second blood sample taken at 48h we found statistically significant differences in haemoglobin, haematocrit, and ferritin levels, with higher levels the longer clamping was delayed. The differences in the levels of haemoglobin and haematocrit were observed in clamping performed as early as minute one (Group 2), and significant differences in ferritin were observed starting at minute 2 (Group 3). These results are consistent with those of other published studies, and support the notion that newborns in whom clamping is delayed start out with a greater red blood cell mass, higher haemoglobin concentrations, and fuller iron stores, better preparing them for infancy.
It is also worth noting that, contrary to what we expected to find, the levels of bilirubin were similar in all 3 groups.
We should also underscore the exclusion criteria in our study, and that there are some patient subsets at greater risk of polycythaemia (those with intrauterine growth restriction, severe placental insufficiency, or born large for gestational age). Therefore clear and safe protocols are needed to perform clamping at the best possible time depending on the specific circumstances.
ConclusionsBased on the results of this study, consistent with those of other publications, we conclude that the rate of clinical complications in newborns does not increase in relation to the different timings of cord clamping. Although we found statistically significant differences in the prevalence of polycythaemia (there were more cases in the late clamping group), the higher prevalence did not lead to the establishment of any special treatment nor any appreciable clinical changes.
We can also conclude that newborns in whom cord clamping was delayed had statistically significant higher levels of haematocrit, haemoglobin, and ferritin at 48h. This may entail a better iron status in the newborn, which may be beneficial throughout infancy and therefore to the newborn's future.
Conflicts of interestsThe authors have no conflicts of interest to declare.
The authors thank Dr. Joan Carles Trullàs i Vila for collaborating in the statistical analysis of the results, as well as the entire team of midwives and nurses that participated in the study.
Please cite this article as: Rincón D, Foguet A, Rojas M, Segarra E, Sacristán E, Teixidor R, et al. Tiempo de pinzamiento del cordón umbilical y complicaciones neonatales, un estudio prospectivo. An Pediatr (Barc). 2014;81:142–148.