Preterm newborns (PN) have a higher risk of thyroid dysfunction than term newborns (TN). This condition may go unnoticed in neonatal screening due to a late elevation of thyrotropin (TSH) in these patients.
ObjectiveEvaluate thyroid function in the second week of life in PN of < 32 weeks gestation (WG), and to identify factors associated to its alteration.
Patients and methodsA retrospective study was performed in neonates of < 32 weeks gestation (WG), in whom thyroid function was determined. An analysis was performed on thyroxine (T4L) and TSH levels, as well as their association with perinatal and neonatal outcomes.
ResultsThe study included a total of 358 patients with mean gestational age (GA) of 29.3 weeks, and mean birth weight (BW) 1127 grams. A linear correlation was found between T4L and BW (correlation coefficient (R) 0.356; p < 0.001) and GA (R = 0.442; p < 0.001). TSH values were associated with small for gestational age (SGA 5.3 mU/L [1.5-37]; non-SGA 2.89 mU/L [0.2-19.5]; p < 0.001), inotropic support (Yes 3.98 mU/L [0.6-22.9]; No 3.16 mU/L [0.2-37]; p = 0.019) and BW (R = -0.249; p < 0.001). Nine (2.5%) patients were treated with levothyroxine, of whom six were SGA.
ConclusionsThyroid function analysis in the second week of life helps to identify asymptomatic newborns with risk of thyroid dysfunction. SGA newborns are at higher risk of thyroid function alterations.
El recién nacido (RN) prematuro (RNPT) tiene mayor riesgo de disfunción tiroidea que el recién nacido a término (RNAT). Esta alteración puede pasar desapercibida en el cribado neonatal por una elevación tardía de tirotropina (TSH) en estos pacientes.
ObjetivoEvaluar la función tiroidea en la segunda semana de vida en RNPT menores a 32 semanas de gestación (SG) e identificar factores asociados con la alteración de esta.
Pacientes y métodosEstudio restrospectivo que incluye RNPT de igual o menos de 32 SG, a los que se realizó función tiroidea. Se analizaron los valores de tiroxina (T4L) y TSH y su relación con variables perinatales y de evolución neonatal.
ResultadosSe presentaron 358 pacientes con edad gestacional (EG) mediana de 29,3 semanas y peso al nacimiento (PN) de 1.127 gramos. Se encontró correlación lineal entre T4L y el PN (coeficiente de correlación (R) 0,356; p < 0,001) y la EG (R = 0,442; p < 0,001). Los valores de TSH se asociaron con ser pequeño para la edad gestacional (PEG 5,3 mU/L [1,5 a 37]; no PEG 2,89 mU/L [0,2 a 19,5]; p < 0,001), al soporte inotrópico (Sí 3,98 mU/L [0,6 a 22,9]; No 3,16 mU/L [0,2 a 37]; p = 0,019) y al PN (R = -0,249; p < 0,001). Recibieron tratamiento sustitutivo con levotiroxina nueve pacientes (2,5%), seis de los cuales fueron PEG.
ConclusionesEl análisis de la función tiroidea en la segunda semana de vida permite identificar RNPT asintomáticos con riesgo de presentar alteración de la función tiroidea. Los RN PEG tienen un riesgo más elevado de disfunción tiroidea.
Thyroid hormones are required for normal growth and maturation of the central nervous system (CNS) and for bone, lung and cardiac maturation in the foetal and neonatal periods.1–3
The increased survival of preterm (PT) infants in recent years has elicited an increased interest in researching certain diseases associated with prematurity, including thyroid dysfunction.
The incidence of thyroid dysfunction is relatively greater in PT infants compared to term infants and increases with decreasing gestational age (GA) and birth weight (BW) and increasing neonatal severity.3–5
The aetiology of thyroid dysfunction in PT infants remains unclear, and potential contributing factors include the interruption of the placental passage of thyroxine from the mother to the foetus, the immaturity of the hypothalamic-pituitary-thyroid axis and decreased synthesis and metabolism of thyroid hormones, inadequate intake of iodine through maternal milk or formula and the increased susceptibility of PT infants to iodine overload.6–8 Thyroid function abnormalities have also been associated with intrauterine growth restriction (IUGR), small size for gestational age (SGA), meconium-stained amniotic fluid, male sex and exposure to drugs such as caffeine, dopamine or steroids.5,9
Low levels of thyroid hormones during a critical period for neural development could have a negative impact on cognitive and psychomotor outcomes in these patients, who are already at risk due to prematurity and its associated complications.
Different abnormalities in thyroid function have been described in PT infants, including hypothyroxinaemia of prematurity (characterized by low levels of free thyroxine [FT4] combined with normal or decreased levels of thyroid-stimulating hormone [TSH]), the primary congenital hypothyroidism (permanent or transient) and central or hypothalamic-pituitary hypothyroidism.6,10
Transient congenital hypothyroidism and hypothyroxinaemia of prematurity are more frequent in PT than in term infants, while the incidence of permanent congenital hypothyroidism and central hypothyroidism is similar in both groups.4,11–13 Still, thyroid abnormalities may go undetected in the newborn screen performed on heel prick samples obtained between 48 and 72 hours post birth if screening is done by measuring the levels of TSH. The reason is that due to the immaturity of the hypothalamic-pituitary axis and the potential influence of neonatal disorders and their treatment, TSH elevation may be delayed in PT infants.7,13–15
Many health care institutions and scientific societies recommend a second screening between weeks 2 and 4 post birth, even if the results of the initial heel prick test are normal, to ensure that affected patients do not go undetected and miss the chance to receive adequate treatment.15–18 For some authors, the usefulness of this screening is debatable, as most patients identified at this point have mild hypothyroidism that is usually also transient and resolves within a few months, so that the need for treatment is also controversial. However, there have also been reports of cases of permanent congenital hypothyroidism in PT infants with a normal heel prick test and diagnosed at a later time.15,19,20 On the other hand, the authors that advocate for screening at a later time are not clear as to the optimal timing of screening or the cut-off T4 and TSH values to be used to determine eligibility for treatment.5,7,13–16
The aim of our study was to assess thyroid function in the second week of life in a sample of PT neonates, assess the usefulness of screening and identify factors associated with abnormal thyroid function.
Sample and methodsWe conducted a retrospective, observational, descriptive and analytical study by including all PT infants delivered at or before 32 weeks of gestation admitted to the neonatal intensive care unit of the Hospital Universitario Vall d’Hebron over a period of 3 years. We included infants born between January 1, 2015 and December 31, 2017 to be able to follow-up the cases through at least age 2 years.
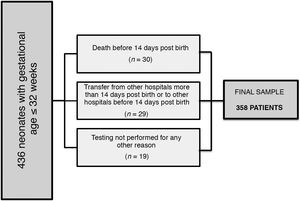
We excluded patients that died before the first thyroid function screening, patients transferred to our unit from other hospitals more than 14 days post birth or from our hospital to other hospitals before 2 weeks post birth, and patients who, for any reason, did not undergo screening within the time frame established in the protocol (mainly neonates with haemodynamic instability or severe respiratory failure).
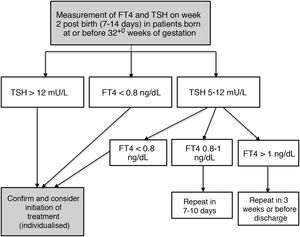
The protocol for monitoring thyroid function in our hospital involves measuring TSH and FT4 levels in the second week of life (7-14 days post birth) in every patient born at 32+0 weeks of gestation or earlier through a routine blood test performed in the morning on weekdays. Additional measurements are performed if the results of initial testing are abnormal based on the cut-off values established in the protocol (Fig. 1).
Blood samples were obtained by venepuncture and processed in the hormone laboratory of the hospital.
The decision to treat was made in collaboration with the paediatric endocrinology unit, providing treatment to patients with persistent TSH elevation greater than 12 mU/L or a FT4 level under 0.8 ng/dL. Treatment consisted in the administration of oral levothyroxine (unless this route was absolutely contraindicated) starting with a dose of 4 to 6 µg/kg/day.
The paediatric endocrinology unit follows up every patient discharged under levothyroxine therapy, and, based on the clinical and laboratory features observed during the follow-up, determined the duration of the treatment and the possibility of discontinuing ambulatory treatment.
We retrieved data from the electronic health records of the patients using the SAP and Centricity Critical Care software.
We collected information on variables related to the antenatal and immediate postpartum periods (type of pregnancy [singleton or twin], sex, cause of delivery, antenatal administration of steroids and magnesium sulphate, gestational age, anthropometric measurements at birth, Apgar score, type of resuscitation in delivery room and administration of surfactant), neonatal outcomes (respiratory and haemodynamic support, intraventricular haemorrhage, early- and late-onset sepsis, death) and serial measurements of thyroid hormones (date of testing, TSH and FT4 levels), as well as prescription of levothyroxine, the date of initiation and, if applicable, the date of discontinuation of this treatment.
We defined SGA as a birth weight and/or length more than 2 standard deviations below the mean.
The study was approved by the committee of research with medicines and the committee of research projects of the Hospital Universitario Vall d’Hebron.
Statistical analysisWe entered all the data to a database and analysed it using the statistical software SPSS Statistics 250 (IBM®) for Mac.
We performed a descriptive analysis to obtain frequency distributions, measures of central tendency and measures of dispersion.
In the inferential analysis, we used the Kolmogorov-Smirnov and Shapiro-Wilk tests to assess whether quantitative data followed a normal distribution, and compared quantitative variables in the different groups using the Student t test and the Mann-Whitney U test as applicable. For qualitative variables, we used the χ2 test or the Fisher exact test.
ResultsSample characteristicsFig. 2 presents a flowchart of the sample selection process. The final sample included 358 patients (177 female and 181 male) with a median GA of 29.3 weeks (23.7-32) and a median BW of 1127 grams (380-2650).
Table 1 summarises the prenatal characteristics and neonatal outcomes of the patients.
Sample characteristics.
| n (%) | |
|---|---|
| Multiple gestation | 113 (31.6%) |
| Gestational age < 28+0 weeks | 114 (31.8%) |
| Birth weight < 1000 grams | 127 (35.5%) |
| Small for gestational age | 61 (17%) |
| Antenatal steroids (2 or more doses) | 275 (76.8%) |
| Intubation in delivery room | 72 (20.1%) |
| Need of surfactant | 162 (45.3%) |
| Need of mechanical ventilation | 128 (35.8%) |
| Need of CPAP | 328 (91.6%) |
| Haemodynamically significant patent ductus arteriosus | 85 (23.7%) |
| Administration of dopamine | 59 (16.5%) |
| Vertical sepsis | 37 (10.3%) |
| Necrotising enterocolitis (any grade) | 27 (7.5%) |
| Nosocomial sepsis | 73 (20.4%) |
| Intraventricular haemorrhage (any grade) | 95 (26.5%) |
| Severe intraventricular haemorrhage (grade III, intraparenchymal haemorrhage) | 15 (4.2%) |
| Death | 10 (2.8%) |
CPAP, continuous positive airway pressure.
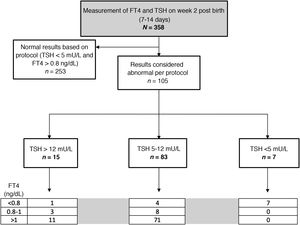
In the tests performed between 7 and 14 days post birth, the median TSH level was 3.25 mU/L (0.2-37), while the median FT4 level was 1.22 ng/dL (range, 0.51-2.2). Based on the threshold values established in the protocol, the blood test was repeated due to abnormal findings in the first in 105 patients (29.3%). The time elapsed between both tests varied based on the magnitude of the alteration in the initial test, with a median time of 15 days (24 hours-45 days).
Fig. 3 presents the distribution of the patients based on the ranges established in the protocol.
Factors involved in thyroid functionWe found that FT4 levels were associated significantly with BW (correlation coefficient, 0.356; P < .001) and GA (correlation coefficient, 0.442; P < .001). We did not find statistically significant differences based on any of the other variables under study.
The levels of TSH were significantly associated with the history of SGA (5.3 mU/L [1.5-37] in SGA infants vs 2.89 mU/L [0.2-19.5] in infants not SGA; P < .001); the need of inotropic support with dopamine (3.98 mU/L [0.6 a 22.9] in treated group vs 3.16 mU/L [0.2 a 37] in untreated group; P = .019) and BW (correlation coefficient, –0.249; P < .001). We did not find an association between TSH values and the rest of the variables analysed, including GA.
Table 2 summarises the thyroid function test results of the patients by GA and BW.
Thyroid-stimulating hormone and free thyroxine values at 7 to 14 days post birth by gestational age and birth weight, expressed as median (minimum-maximum).
| Gestational age | < 26 weeks | 26+0 to 27+6 weeks | 28+0 to 29+6 weeks | ≥ 30 weeks |
|---|---|---|---|---|
| n | 41 | 73 | 92 | 152 |
| TSH (mU/L) | 3.5 (0.59 - 19.5) | 3.4 (0.74 - 13.6) | 3.6 (0.2 - 22.9) | 2.9 (0.51 - 37) |
| P5-P95 | 0.65 - 16.1 | 1 - 10.5 | 1.15 - 13.3 | 1.18 - 11.5 |
| FT4 (ng/dL) | 1.01 (0.51 -1.66) | 1.12 (0.72 - 1.9) | 1.22 (0.82 - 1.9) | 1.32 (0.73 -2.25) |
| P5-P95 | 0.68 - 1.48 | 0.81 - 1.56 | 0.84 - 1.61 | 0.94 - 1.72 |
| Birth weight | < 750 g | 750 - 999 g | 1000 - 1249 g | ≥1250 g |
| n | 49 | 78 | 88 | 143 |
| TSH (mU/L) | 4.3 (0.59 -22.9) | 3.9 (0.65 - 37) | 3.8 (0.74 - 12.8) | 2.4 (0.2 -17.4) |
| P5-P95 | 0.72 - 18 | 0.88 - 12.5 | 1.2 - 9.7 | 1.1 - 8.8 |
| FT4 (ng/dL) | 0.99 (0.51 -1.66) | 1.1 (0.68 - 2.03) | 1.2 (0.75 - 1.9) | 1.3 (0.73 -2.25) |
| P5-P95 | 0.69 - 1.57 | 0.74 - 1.6 | 0.83 - 1.6 | 0.94 - 1.73 |
FT4, free thyroxine; P5-P95, 5th-95th percentiles; TSH, thyroid-stimulating hormone.
Treatment with oral levothyroxine was prescribed to 9 patients (2.5%). In 3, treatment started after the results of the first test became available, following confirmation with an early follow-up test within 24 to 48 hours (in 2, due to TSH levels greater than 20 mU/L despite normal levels of FT4, and in 1 due to hypothyroxinaemia in the context of septic shock). In 2 PT infants, treatment was initiated after the second test due to hypothyroxinaemia with marked elevation of TSH, and in 3 due to persistent elevation of TSH despite normal FT4 levels in subsequent tests. The remaining patient started treatment at 2 months post birth in the context of nephrotic syndrome after having had normal results in the initial screening.
Table 3 presents the characteristics of the patients treated with levothyroxine.
Anthropometric and laboratory characteristics of patients treated with levothyroxine.
| Sex | BW | GA | Newborn screening | TSH 7-14 days | FT4 7-14 days | TSH at treatment initiation | FT4 at treatment initiation | Time of treatment initiation (days post birth) | Discontinuation of treatment (months of life) | Other characteristics | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days post birth | Abnormal | TSH (mU/L) | |||||||||||
| 1 | F | 1000 | 32+0 | 3 | No | 11.6 | 0.78 | 186.1 | 0.35 | 24 | Yes (9) | SGA | |
| 2 | F | 735 | 29+2 | 4 | No | 3.00 | 15.4 | 1.60 | 39.8 | 0.94 | 20 | Yes (33) | SGA |
| 3 | F | 645 | 30+5 | 5 | No | 18.8 | 1.28 | 16.3 | 1.3 | 35 | Yes (21) | SGA | |
| 4 | M | 1600 | 29+6 | 5 | No | 4.20 | 17.4 | 1.48 | 20.8 | 1.2 | 18 | No | Mosaicism trisomy 21 |
| 5 | M | 825 | 30+0 | 4 | No | 5.30 | 37 | 0.94 | 39.2 | 0.94 | 12 | No | SGA |
| 6 | M | 880 | 29+2 | 4 | No | 1.70 | 14.8 | 0.88 | 94.7 | 0.65 | 18 | No | SGA, Williams syndrome |
| 7 | F | 600 | 29+4 | 13 | Yes | 13.00 | 22.9 | 1.29 | 23.4 | 1.29 | 14 | Yes (12) | SGA, dopamine |
| 8 | M | 1000 | 28+4 | 3 | No | 5.3 | 1.20 | 15.1 | 1.05 | 60 | Yes (14) | Nephrotic syndrome, dopamine | |
| 9 | M | 650 | 24+0 | 3 | No | 19.5 | 0.68 | 18.6 | 0.67 | 15 | 30 days | Septic shock on week 2 | |
BW, birthweight (grams); F, female; FT4, free thyroxine (ng/dL); GA, gestational age (weeks + days); M, male; SGA, small for gestational age; TSH, thyroid-stimulating hormone (mU/L).
We found a statistically significant association between the indication of treatment with levothyroxine and BW (825 g [600-1600] in treated group; 1137 g [380-2650] in untreated group; P = .011) and the history of SGA (treatment in 11.7% of SGA infants vs 0.7% of not SGA infants; P < .001). We did not find an association between the prescription of pharmacological treatment and any of the other variables under study. The findings of the ultrasound examination of the thyroid were normal in all patients that received levothyroxine.
Treatment was only discontinued during the stay in the patient with septic shock upon detecting inhibition of TSH with high FT4 levels. In the follow-up conducted at the outpatient paediatric endocrinology clinic, levothyroxine was suspended before age 3 years in 5 patients (median, 14 months; range, 9-33 months). Two patients continue to receive levothyroxine: 1 with trisomy 21 and 1 with Williams syndrome confirmed by genetic testing after discharge. One other patient was lost to follow-up after moving to a different province.
DiscussionThe increased survival of PT infants has allowed the identification of disorders other than those typically described in the past literature, including abnormalities in thyroid function.7,17,21,22
Many studies have provided reference values and analysed thyroid function in paediatric patients, but there are fewer data on the values found in PT infants. This case series is one of the largest assessing thyroid function in extremely PT neonates in recent years.18,23,24
Hypothyroxinaemia of prematurity, characterized by reduced T4 levels without elevation of TSH, has been found to be relatively frequent in neonates born before 32 weeks’ gestation, with frequencies as high as 50% reported in infants delivered before 28 weeks.22,25 The FT4 threshold established by the Sociedad Española de Endocrinología Pediátrica (Spanish Society of Paediatric Endocrinology) is 0.8 ng/dL, compared to a cut-off value of 0.7 ng/dL in international guidelines.8,18,25,26 However, in our sample, contrary to the previous literature, we only found hypothyroxinaemia in 3.3% of patients, and FT4 levels had normalised in the second screening in all of them except the 3 PT infants that received treatment for this condition. We did not identify any cases of central hypothyroidism.
In our study, patients with a lower BW and GA had lower levels of FT4, which was consistent with the literature. Birth weight was also associated with TSH values (although the correlation was weak) and with the history of SGA, but we did not find a statistically significant association between TSH values and GA. A more thorough analysis of these outcomes revealed that patients with low BWs and elevation of TSH tended to have a history of SGA and had been born at a higher GA, so it is possible that these infants had a more mature hypothalamic-pituitary axis compared to infants with similar BWs and lower GAs.9,27
We also found an association between TSH levels and the administration of dopamine. Previous studies have found slightly elevated levels of TSH at 14 days post birth in neonates that had recovered from some form of severe disease in the first 2 weeks of life and that had received inotropes or steroids, which could be indicative of recovery from an episode of transient central hypothyroidism.3,22
In our sample, most of the patients that received levothyroxine were SGA (GA ≥ 28 weeks and BW ≤ 1000 g), the exceptions being the patients with genetic disorders and the PT infant with septic shock in whom levothyroxine could be discontinued after 2 weeks. In agreement with previous studies, we found a higher risk of thyroid dysfunction in SGA infants compared to appropriate for GA infants.
The frequency of thyroid dysfunction for which treatment was indicated in extremely low birth weight patients (4.7% in our sample) was similar or slightly higher compared to previous case series.7,13,17
With one exception, none of the patients treated with levothyroxine had abnormal results in the initial newborn screening, which means that thyroid dysfunction in these patients would not have been diagnosed if it had not been for the screening protocol applied in the study. In the one patient with abnormal results in the initial screening, testing was delayed to 13 days post birth on account of septic shock in the first days of life that required multiple transfusions of blood products, so that the week 2 blood test included in the protocol was also useful for the early identification of that anomaly, and the patient was already receiving treatment by the time the abnormal results of the heel prick test became available.
These results corroborate the importance of performing a second screening in patients born PT or with risk factors for hypothyroidism, as it allows identification of newborn infants with abnormal thyroid function that cannot be detected in the newborn screening performed at 48 to 72 hours post birth due to delayed elevation of TSH.16,17
There is significant disagreement as regards the short- and long-term consequences of a relative deficiency of thyroid hormones in PT neonates, the optimal ranges for adequate maturation and the need for hormone replacement in these patients.2,25,26
While some authors have described poorer neurodevelopmental outcomes in patients with a history of prematurity and hypothyroxinaemia in the neonatal period,2,28–30 others have found no differences in psychomotor and cognitive outcomes in these infants compared to PT infants with normal thyroid function in the neonatal period, including some with long follow-ups to up to age 19 years, such as the study published by Hollanders in 2015.31–34
On the other hand, there is little evidence to date on the repercussions of replacement with levothyroxine from clinical trials or prospective studies. Some authors question whether transient congenital hypothyroidism or hypothyroxinaemia of prematurity should be treated, given that they tend to be mild and temporary in most patients6,15,17,19 and that the literature published to date does not demonstrate a clear beneficial effect on long-term neurodevelopmental outcomes. However, the evidence is yet insufficient to determine whether treatment with thyroid hormones could reduce the incidence of neurologic sequelae in these patients.20,25,35,36
Given the importance of adequate thyroid function in a period so critical to the development of the central nervous system, it seems prudent to initiate treatment in patients with persistent hypothyroxinaemia or hyperthyrotropinaemia, always after confirming test results, avoiding initiation of treatment based on a single isolated value and assessing the possibility of discontinuation before age 3 years, as described by some authors and recommended by the most recent guidelines.18,22,25
In our sample, it was possible to discontinue treatment before age 3 years in every patient with the exception of children with genetic disorders (trisomy 21 and Williams syndrome), in whom thyroid dysfunction was actually missed in the initial heel prick test.
In conclusion, we believe that measurement of thyroid hormone levels on week 2 post birth allows the identification of PT neonates at risk of thyroid abnormalities that may not be detected in the newborn screening. Given the absence of universally accepted cut-off values to determine eligibility for treatment, each hospital should establish them based on an assessment of outcomes in its patients, and it seems prudent to initiate treatment in the case of persistent abnormalities and assess whether it can be discontinued in the first years of life, except in patients with specific diseases associated with an increased risk of permanent congenital hypothyroidism.
Given the lack of clear evidence, prospective, randomised, placebo-controlled trials are needed to assess the short- and long-term benefits of replacement therapy with levothyroxine in PT infants with thyroid dysfunction.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Montaner-Ramón A, Hernández-Pérez S, Campos-Martorell A, Ballesta-Anguiano M, Clemente-León M, Castillo-Salinas F. Función tiroidea en el recién nacido prematuro con edad gestacional igual o menor a 32 semanas. An Pediatr (Barc). 2022;96:130–137.
Previous presentation: The study was presented as an oral communication at the XXVI Congress of Neonatology and Perinatal Medicine of the Sociedad Española de Neonatología; September 2017, Zaragoza, Spain.