Neonatal herpes simplex virus infections are rare, but are associated with significant morbidity and mortality. Most newborns acquire herpes simplex virus infection in the peripartum period. For peripartum transmission to occur, women must be shedding the virus in their genital tracts symptomatically or asymptomatically around the time of delivery. There are evidence-based interventions in pregnancy to prevent the transmission to the newborn. Caesarean section should be performed in the presence of herpetic lesions, and antiviral prophylaxis in the last weeks of pregnancy is recommended to suppress genital tract herpes simplex virus at the time of delivery. The diagnosis and early treatment of neonatal herpes simplex virus infections require a high index of suspicion, especially in the absence of skin lesions. It is recommended to rule out herpes simplex virus infections in those newborns with mucocutaneous lesions, central nervous system involvement, or septic appearance. The prognosis of newborns with skin, eye, and/or mouth disease in the high-dose acyclovir era is very good. Antiviral treatment not only improves mortality rates in disseminated and central nervous system disease, but also improves the rates of long-term neurodevelopmental impairment in the cases of disseminated disease. Interestingly, a 6-month suppressive course of oral acyclovir following the acute infection has improved the neurodevelopmental prognosis in patients with CNS involvement.
La infección herpética neonatal es una entidad muy poco frecuente pero que se asocia a una alta morbimortalidad. La mayor parte de los neonatos afectos adquieren la infección por virus herpes simplex en el periodo periparto. Para que ocurra esta transmisión es necesaria la excreción viral genital, con o sin síntomas, alrededor del momento del parto. Existen intervenciones basadas en la evidencia para prevenir la transmisión del virus herpes simplex al recién nacido. La realización de una cesárea en presencia de lesiones herpéticas, y la disminución de la excreción viral administrando en las últimas semanas del embarazo tratamiento antiviral a gestantes con herpes genital activo, son las mejores medidas preventivas de las que se dispone. El diagnóstico y tratamiento precoz del herpes neonatal requiere de un alto índice de sospecha, sobre todo en ausencia de lesiones cutáneas. Se recomienda descartar la infección por herpes neonatal en aquellos recién nacidos con lesiones cutaneomucosas, afectación del sistema nervioso central o cuadro séptico de origen no aclarado. El pronóstico de los neonatos con enfermedad cutánea en la era del aciclovir a dosis altas es excelente. El tratamiento antiviral disminuye la mortalidad de las formas diseminadas y con afectación exclusiva del sistema nervioso central, pero también mejora el pronóstico neurológico en los casos de enfermedad diseminada. De forma notable, la introducción del tratamiento supresor con aciclovir oral durante los meses siguientes a la infección aguda ha mejorado el pronóstico neurológico en los pacientes con afectación del sistema nervioso central.
Infection by herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) is highly prevalent worldwide and encompasses a broad range of pathology. Up to 67% of the global population is infected by one of these two viruses.1
The first episode of genital infection is labelled primary genital herpes (GH) and may be caused by HSV-1 or HSV-2. When an additional episode is caused by the other type, it is referred to as non-primary first episode GH. New episodes by the same type of the virus are known as recurrent GH.
These viruses may be vertically transmitted to the newborn. Neonatal herpes infection (neonatal herpes [NH]) is rare, but it must be diagnosed correctly and treated at an early stage. In recent years, there have been advances in the diagnosis and treatment of this disease, so this document provides and updated guideline for its multidisciplinary management.
EpidemiologyGenital herpesThe type involved most frequently in GH worldwide is HSV-2. The prevalence of HSV-1 is increasing in many countries, such as the United States, where it is now the most frequent cause of new cases of GH.2
In Spain, the incidence of genital infections by HSV has increased in recent years.3 Type 2 has been historically the type isolated most frequently from genital samples,4 a trend that continues today.3,5 The seroprevalence of HSV-2 infection in adults is 5–10%.3,5
The risk factors for GH include: female sex, low socioeconomic status, genital coinfection, years of sexual activity and large number of sexual partners. Based on data from the medical literature of the United States, the overall risk of having a first episode of GH during pregnancy is 4%.6 Also, of all pregnant women with a previous history of symptomatic GH by HSV-2, 75% have at least one episode of recurrent GH during pregnancy.7 The risk of recurrence is lower in women with GH caused by HSV-1.
The identification of NH is frequently challenging. In up to 80% of cases of mother-to-child vertical transmission, there is no previous history of GH,6 although genital viral shedding around the time of delivery, whether symptomatic or asymptomatic, is necessary for transmission. Between 0.2% and 0.39% of pregnant women shed HSV in the genital region during the peripartum period, independently of their personal history of GH, and this prevalence increases to 0.77–1.4% in women with a history of recurrent GH.7 Viral shedding usually lasts 2 or 3 weeks after a primary GH episode, although it can persist for up to 2 or 3 months, is shorter in non-primary GH episodes, and intermittent in recurrent episodes.
Neonatal herpesNeonatal herpes is rare in developed countries, with an incidence that ranges between 1.65 and 3.2 cases per 100000 live births in European countries like the Netherlands or Switzerland.7 In other countries there has been a recent increase in incidence, for instance, the incidence in the United Kingdom recently reached 17.5 cases per 100000 births.8 For reasons that remain unclear, the incidence of NH has historically been greater in the United States compared to the European average, ranging between 4 and 33 cases per 100000 live births.6
Both HSV-1 and HSV-2 cause disease in the neonatal period, although the proportion of cases caused by either virus varies between case series, reflecting the epidemiology of GH in the geographical area or the time period of the study. Type 2 is the most frequent cause of NH in developing countries.1 The incidence of HSV-1 has increased in developed countries, and HSV-1 is now the most prevalent cause of NH in some countries, including Australia and the United States.9
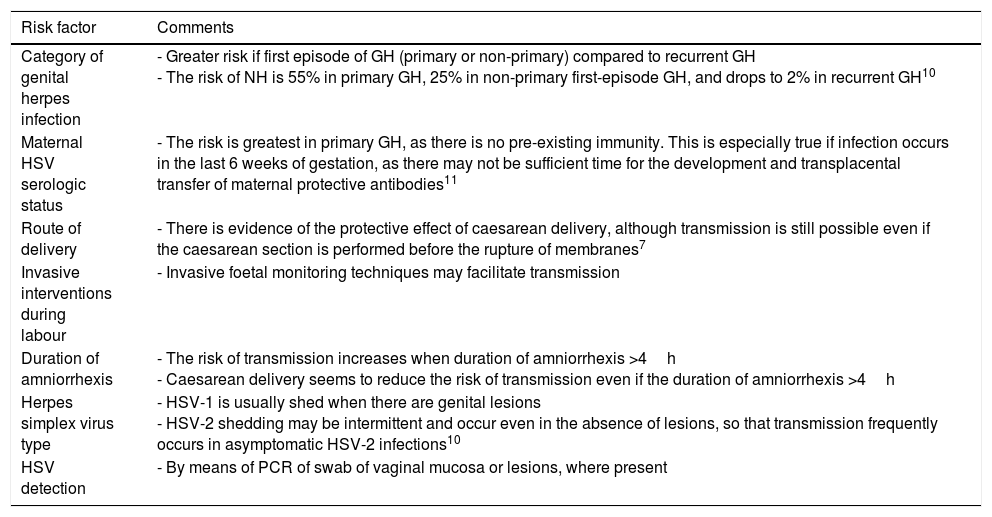
Table 1 presents the risk factors for NH.
Risk factors for neonatal herpes.
| Risk factor | Comments |
|---|---|
| Category of genital herpes infection | - Greater risk if first episode of GH (primary or non-primary) compared to recurrent GH - The risk of NH is 55% in primary GH, 25% in non-primary first-episode GH, and drops to 2% in recurrent GH10 |
| Maternal HSV serologic status | - The risk is greatest in primary GH, as there is no pre-existing immunity. This is especially true if infection occurs in the last 6 weeks of gestation, as there may not be sufficient time for the development and transplacental transfer of maternal protective antibodies11 |
| Route of delivery | - There is evidence of the protective effect of caesarean delivery, although transmission is still possible even if the caesarean section is performed before the rupture of membranes7 |
| Invasive interventions during labour | - Invasive foetal monitoring techniques may facilitate transmission |
| Duration of amniorrhexis | - The risk of transmission increases when duration of amniorrhexis >4h - Caesarean delivery seems to reduce the risk of transmission even if the duration of amniorrhexis >4h |
| Herpes simplex virus type | - HSV-1 is usually shed when there are genital lesions - HSV-2 shedding may be intermittent and occur even in the absence of lesions, so that transmission frequently occurs in asymptomatic HSV-2 infections10 |
| HSV detection | - By means of PCR of swab of vaginal mucosa or lesions, where present |
GH, genital herpes; HSV, herpes simplex virus; NH, neonatal herpes; PCR, polymerase chain reaction.
The diagnosis of GH in pregnant women is based on history taking and a physical examination, and it must be confirmed by laboratory tests.
Clinical pictureThe clinical picture is characterised by the formation of lesions (vesicles that progress to pustules and eventually ulcers), usually painful, in the genital tract and adjacent areas. Primary infection may manifest with general malaise, dysuria and inguinal lymphadenopathy. However, the first episode of GH may be asymptomatic in up to 2/3 of women. The presentation of recurrent GH is milder, although it is usually preceded by prodromal manifestations such as genital itching, burning or pain.
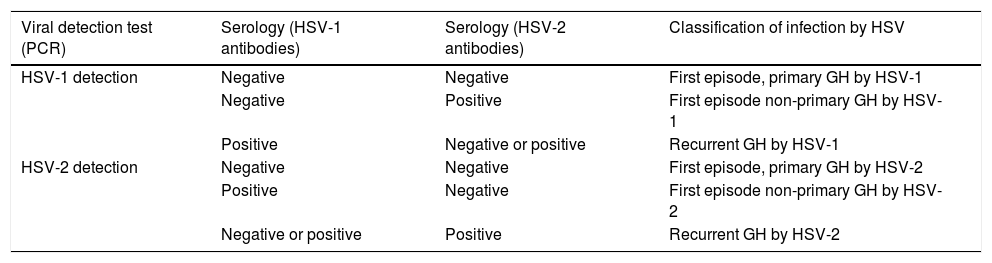
Laboratory diagnosisFirst episode of genital herpes during pregnancyFirst episodes of GH can be primary or non-primary. Viral detection tests by polymerase chain reaction (PCR) should be performed in samples of ulcer secretions or vesicle fluid collected by swabbing. Polymerase chain reaction is sensitive enough for testing of samples from any type of lesion and is currently the gold standard where available.12 The technique used should be type-specific PCR (for HSV-1 and -2). Serological tests for HSV (specific antibodies to HSV-1 and 2) at the time of diagnosis allows the classification of maternal GH infection into primary, first-episode non-primary or recurrent infection (Table 2).13 It may take up to 6 weeks from primary infection for IgG antibodies to become detectable. Serological testing for detection of IgM antibodies is not recommended in clinical practice.
Classification of the type of genital herpes based on the results of lesion swab viral detection tests and serologic tests (type-specific).
| Viral detection test (PCR) | Serology (HSV-1 antibodies) | Serology (HSV-2 antibodies) | Classification of infection by HSV |
|---|---|---|---|
| HSV-1 detection | Negative | Negative | First episode, primary GH by HSV-1 |
| Negative | Positive | First episode non-primary GH by HSV-1 | |
| Positive | Negative or positive | Recurrent GH by HSV-1 | |
| HSV-2 detection | Negative | Negative | First episode, primary GH by HSV-2 |
| Positive | Negative | First episode non-primary GH by HSV-2 | |
| Negative or positive | Positive | Recurrent GH by HSV-2 |
GH, genital herpes; HSV, herpes simplex virus; PCR, polymerase chain reaction.
In women with genital ulcers that are highly suggestive of GH but with negative HSV PCR results, antibody testing should be repeated 6 weeks later. If testing evinces seroconversion, the episode should be diagnosed as primary infection. If testing does not reveal seroconversion, it is unlikely that the ulcer was caused by GH.
Pregnant women with recurrent genital herpesIn women with a previous history of confirmed GH (PCR), further diagnostic testing is unnecessary. There is no evidence that repeated testing by PCR to detect asymptomatic shedding during pregnancy or at the time of delivery helps prevent vertical transmission, and this strategy is therefore not indicated.14
However, if a pregnant woman has compatible lesions and reports a history of previous episodes but these episodes were not confirmed by diagnostic tests, performance of PCR and antibody tests is indicated to confirm the diagnosis.
Treatment of herpes simplex virus infection during pregnancy. Strategies for prevention of mother-to-child transmission (Table 3)The main goal of GH treatment during pregnancy is prevention of mother-to-child transmission. The best measures currently available to this end are caesarean delivery in mothers that have herpetic lesions and the reduction of viral shedding by administration of antiviral agents to pregnant women with active GH in the last few weeks of pregnancy. The FDA has classified acyclovir and valacyclovir as category B drugs, but their use is considered safe in any trimester of gestation.15 Since there is less evidence on valacyclovir, some guidelines recommend avoiding this drug in the first trimester.14 Suppressive therapy has proven effective in reducing recurrence and the need for caesarean delivery, although the evidence published to date has not demonstrated a reduction in the risk of NH16 (Table 3).
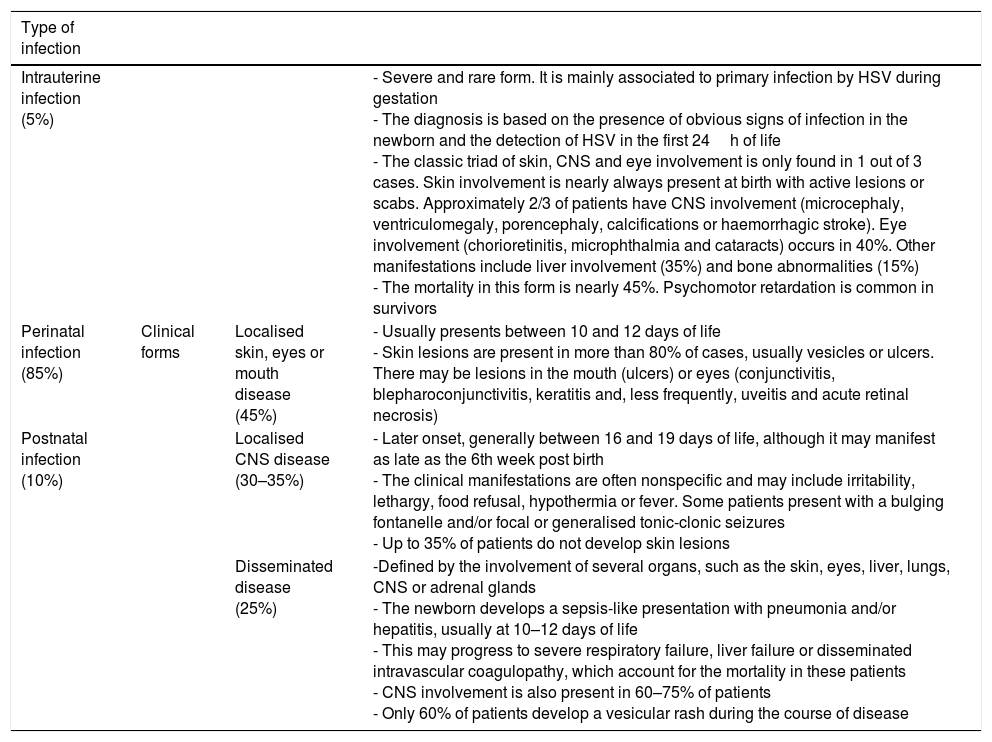
Forms of neonatal infection by HSV.19–25
| Type of infection | |||
|---|---|---|---|
| Intrauterine infection (5%) | - Severe and rare form. It is mainly associated to primary infection by HSV during gestation - The diagnosis is based on the presence of obvious signs of infection in the newborn and the detection of HSV in the first 24h of life - The classic triad of skin, CNS and eye involvement is only found in 1 out of 3 cases. Skin involvement is nearly always present at birth with active lesions or scabs. Approximately 2/3 of patients have CNS involvement (microcephaly, ventriculomegaly, porencephaly, calcifications or haemorrhagic stroke). Eye involvement (chorioretinitis, microphthalmia and cataracts) occurs in 40%. Other manifestations include liver involvement (35%) and bone abnormalities (15%) - The mortality in this form is nearly 45%. Psychomotor retardation is common in survivors | ||
| Perinatal infection (85%) | Clinical forms | Localised skin, eyes or mouth disease (45%) | - Usually presents between 10 and 12 days of life - Skin lesions are present in more than 80% of cases, usually vesicles or ulcers. There may be lesions in the mouth (ulcers) or eyes (conjunctivitis, blepharoconjunctivitis, keratitis and, less frequently, uveitis and acute retinal necrosis) |
| Postnatal infection (10%) | Localised CNS disease (30–35%) | - Later onset, generally between 16 and 19 days of life, although it may manifest as late as the 6th week post birth - The clinical manifestations are often nonspecific and may include irritability, lethargy, food refusal, hypothermia or fever. Some patients present with a bulging fontanelle and/or focal or generalised tonic-clonic seizures - Up to 35% of patients do not develop skin lesions | |
| Disseminated disease (25%) | -Defined by the involvement of several organs, such as the skin, eyes, liver, lungs, CNS or adrenal glands - The newborn develops a sepsis-like presentation with pneumonia and/or hepatitis, usually at 10–12 days of life - This may progress to severe respiratory failure, liver failure or disseminated intravascular coagulopathy, which account for the mortality in these patients - CNS involvement is also present in 60–75% of patients - Only 60% of patients develop a vesicular rash during the course of disease |
CNS, central nervous system; HSV, herpes simplex virus.
- •
Treatment: Oral acyclovir at 400mg every 8h or oral valacyclovir at 1g every 12h for 7–10 days.14,17
- •
Route of delivery: Whenever primary HSV infection is diagnosed during labour, a caesarean delivery will be performed as soon as possible, regardless of the duration of amniorrhexis. An elective caesarean delivery will be performed in every pregnant woman that has a primary episode of GH within 6 weeks from delivery.14,17,18
- •
Treatment: Consider administration of oral acyclovir at 400mg every 8h or oral valacyclovir at 500mg every 12h for 3–5 days. In general, treatment is only necessary if the mother has significant symptoms or if the lesions appear when the time of delivery is approaching.14,17,18
- •
Route of delivery: Elective caesarean delivery is only indicated in patients that have an outbreak or prodromal symptoms during labour, regardless of the duration of amniorrhexis. An episode of GH at any other time of the pregnancy is not an indication for caesarean delivery. In case of vaginal delivery, invasive procedures (such as continuous foetal heart rate monitoring with scalp electrodes or foetal blood microsampling) and prolonged rupture of membranes should be avoided.
In pregnant women with a first episode of GH (primary or non-primary) or with recurrent GH and at least one symptomatic outbreak during pregnancy, suppressive therapy with oral acyclovir at 400mg every 8h or valacyclovir at 500mg every 12h is recommended starting from 36 weeks’ gestation through delivery.14,17,18 In women at risk of preterm delivery, use of suppressive antivirals may be considered at an earlier gestational age when the risk of preterm birth due to short cervix or dynamic changes is identified.18
Women with HIV infection and a history of GH (even those without episodes during gestation) should be offered suppressive therapy starting at 32 weeks’ gestation due to the increased risk of preterm delivery and of HIV transmission associated with HSV infection.14
Genital herpes in pregnant women with premature rupture of membranes at more than 37 weeks’ gestation- •
In cases of maternal primary infection, the risk of NH is very high, but there is little evidence on the optimal management of these cases. The management will be individualised and based on gestational age. If the decision is to end the pregnancy immediately, the child will be delivered by caesarean section. If a watchful waiting approach is chosen, the mother will be given intravenous acyclovir (5mg/kg every 8h) for a maximum of 7–10 days, and suppressive therapy until delivery may be considered. If the time elapsed between onset of GH and delivery is less than 6 weeks, delivery by caesarean section is preferable.14,17
- •
In cases of maternal recurrent infection, the mother will receive oral antiviral therapy and the followup will be the same as would be in case of premature rupture of membranes. If the lesions have not resolved by the time ending the pregnancy is indicated, the child will be delivered by caesarean section.14,17
Table 3 describes the 3 forms of NH associated to perinatal and postnatal infection. This classification has significant implications for treatment, and each of the forms has a different prognosis.
Diagnosis of infection in the newbornThe initial manifestations of NH may be subtle and nonspecific. Its presentation may be confused with other diseases, mainly bacterial infections (sepsis or meningitis) or viral infections (especially infection by enterovirus or parechovirus). Early diagnosis and treatment of NH requires a high level of suspicion, especially in the absence of skin lesions. We recommend ruling out NH infection in newborns with lesions in the skin or mucosae, central nervous system (CNS) involvement or a sepsis-like picture of unexplained aetiology.
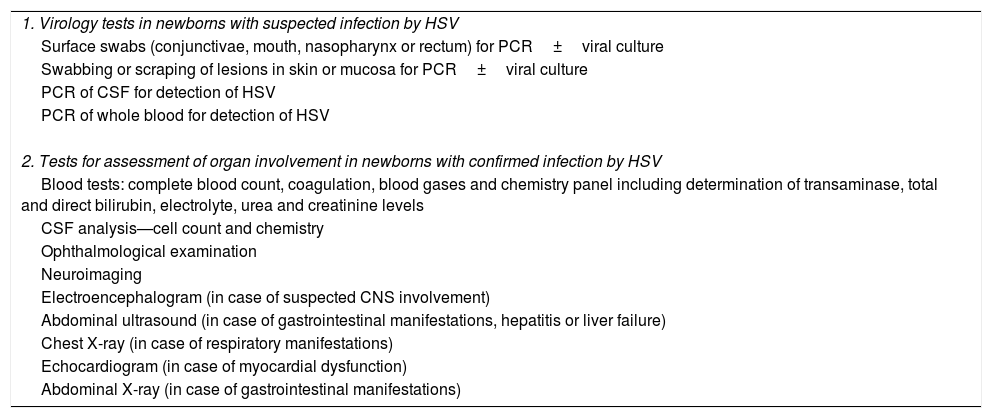
When NH is suspected, the newborn should undergo comprehensive viral testing (Table 4).
Diagnosis and evaluation of neonatal infection by HSV.
| 1. Virology tests in newborns with suspected infection by HSV |
| Surface swabs (conjunctivae, mouth, nasopharynx or rectum) for PCR±viral culture |
| Swabbing or scraping of lesions in skin or mucosa for PCR±viral culture |
| PCR of CSF for detection of HSV |
| PCR of whole blood for detection of HSV |
| 2. Tests for assessment of organ involvement in newborns with confirmed infection by HSV |
| Blood tests: complete blood count, coagulation, blood gases and chemistry panel including determination of transaminase, total and direct bilirubin, electrolyte, urea and creatinine levels |
| CSF analysis—cell count and chemistry |
| Ophthalmological examination |
| Neuroimaging |
| Electroencephalogram (in case of suspected CNS involvement) |
| Abdominal ultrasound (in case of gastrointestinal manifestations, hepatitis or liver failure) |
| Chest X-ray (in case of respiratory manifestations) |
| Echocardiogram (in case of myocardial dysfunction) |
| Abdominal X-ray (in case of gastrointestinal manifestations) |
CNS, central nervous system; CSF, cerebrospinal fluid; HSV, herpes simplex virus; PCR, polymerase chain reaction.
The confirmation of the diagnosis through laboratory methods mainly involves the detection of HSV by PCR.
Polymerase chain reaction for detection of HSV in cerebrospinal fluid (CSF) is the gold standard for diagnosis due to the higher sensitivity of viral culture of this type of sample. In the current literature, the reported sensitivity of PCR for detection of HSV in CSF ranges between 75% and 100%, while the reported specificity between ranges between 71% and 100%.26,27 In clinical practice, false positives for HSV are extremely rare. Polymerase chain reaction for detection of HSV in blood samples is also highly sensitive and is recommended for the diagnostic evaluation of newborns with suspected NH of any form.28
Lumbar puncture should be performed in every newborn with suspected NH, even in cases where isolated skin involvement is likely. The presence of blood or elevated protein levels in CSF may interfere with PCR and lead to false negative results. Polymerase chain reaction may also yield false negative results if lumbar puncture is performed in the early stages of disease (first 3 days) or after several days of antiviral therapy. If the initial PCR test is negative and CNS involvement is suspected, a repeat PCR should be performed in the first week since onset, and antiviral therapy maintained until the results become available.
The characteristic findings of CSF analysis are pleocytosis with a predominance of mononuclear cells and moderate elevation of protein and glucose levels. The findings of CSF analysis may be normal in the early stages of CNS involvement. The presence of red blood cells in CSF does not seem characteristic of HSV-related neonatal meningoencephalitis.
Neuroimaging tests should be performed in every newborn with NH, and the gold standard is magnetic resonance imaging (MRI). Involvement is usually diffuse in newborns with HSV encephalitis. The usual course of CNS involvement is oedema that progresses to cystic encephalomalacia. Neuroimaging findings may be normal at first, and diffusion-weighted MRI is the most sensitive technique for the early detection of CNS changes. Magnetic resonance imaging can be used on completion of antiviral treatment to assess the level of brain damage. The diagnostic yield of computed tomography is lower compared to MRI. Head ultrasound offers the advantage of allowing followup through serial examinations.
Although regular or nearly regular focal or multifocal epileptiform discharges are the typical encephalographic presentation, the encephalographic pattern in the acute phase of neonatal encephalitis due to HSV can include a variety of features.29 The presence of focal or unilateral abnormalities associated to clinical signs of encephalitis is highly suggestive of HSV infection.
The evaluation of the extent of dissemination is key to provide adequate treatment in the early stages of disease (Table 4). Chest radiography may reveal lung involvement, characterised by a diffuse interstitial pattern that may progress to pneumonitis with alveolar haemorrhage.30 In cases of hepatitis, abdominal ultrasound may reveal hepatomegaly with fine nodular patterns in the liver parenchyma, splenomegaly, signs of portal hypertension and ascites.31
TreatmentTreatment of acute infectionTreatment of NH includes the usual life support measures used in any septic process in newborns, management of potential complications (seizures, in which it is essential to avoid hypoglycaemia, disseminated intravascular coagulation, bacterial superinfection, etc.) and antiviral therapy. High-dose acyclovir (20mg/kg every 8h, intravenously, adjusted based on renal function) is the first-line antiviral for all forms of disease.22,24,32
In most cases, clinical suspicion of infection by HSV is sufficient indication for treatment. Early initiation of acyclovir improves morbidity and mortality outcomes for HSV infection. We recommend monitoring renal function and neutrophil counts during treatment. In cases where acyclovir is contraindicated, ganciclovir of foscarnet can be used, also delivered intravenously.
Treatment should last 14 days in the skin, eyes and mouth form and 21 days in cases of localised CNS involvement or disseminated disease. Treatment may be discontinued after the patient shows a favourable response, although in cases of CNS involvement, PCR should be performed to verify negativization, which is usually accompanied by the normalisation of the CNS analysis findings. In case the virus is detected in CSF, treatment with acyclovir will continue accompanied by weekly testing of the CSF, and suspended once the PCR results become negative.32 Some authors consider plasma viral levels undetectable by PCR a marker of resolution in severe or disseminated forms of disease, and continued treatment in their patients as long as viral detection tests were positive.28
In case of keratitis and/or conjunctivitis, the patient should also receive topical treatment (idoxuridine 0.1% solution or ganciclovir 0.15% gel). If the patient has with mucocutaneous lesions, adequate contact precautions should be followed throughout the hospital stay.
Suppressive treatment. Treatment of recurrent herpesFollowing treatment of acute disease, suppressive therapy with acyclovir is recommended for all forms of disease (300mg/m2/dose given orally every 8h) for a minimum of 6 months.32,33 The dose must be adjusted as the patient grows, and the neutrophil count must be monitored, with measurements 2 weeks after treatment initiation and monthly thereafter. Most experts recommend temporary discontinuation of suppressive therapy if the neutrophil count is less than 500cells/mm3.
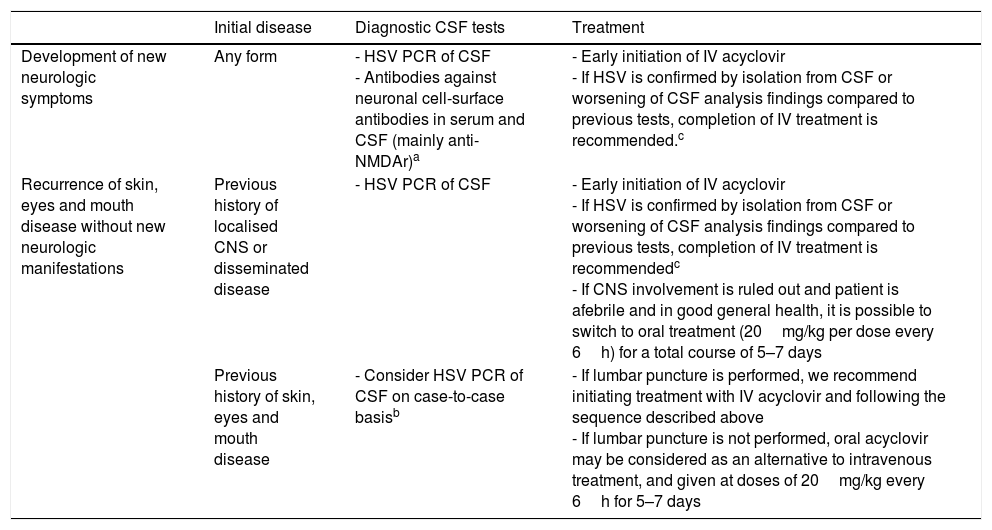
Disease recurrence can even occur in the context of suppressive treatment. The current evidence is not sufficient to establish the best course of action in these cases. Table 5 presents the recommendations of our Working Group.
Recommendations for the management of recurrent disease in patients undergoing suppressive therapy with acyclovir.
| Initial disease | Diagnostic CSF tests | Treatment | |
|---|---|---|---|
| Development of new neurologic symptoms | Any form | - HSV PCR of CSF - Antibodies against neuronal cell-surface antibodies in serum and CSF (mainly anti-NMDAr)a | - Early initiation of IV acyclovir - If HSV is confirmed by isolation from CSF or worsening of CSF analysis findings compared to previous tests, completion of IV treatment is recommended.c |
| Recurrence of skin, eyes and mouth disease without new neurologic manifestations | Previous history of localised CNS or disseminated disease | - HSV PCR of CSF | - Early initiation of IV acyclovir - If HSV is confirmed by isolation from CSF or worsening of CSF analysis findings compared to previous tests, completion of IV treatment is recommendedc - If CNS involvement is ruled out and patient is afebrile and in good general health, it is possible to switch to oral treatment (20mg/kg per dose every 6h) for a total course of 5–7 days |
| Previous history of skin, eyes and mouth disease | - Consider HSV PCR of CSF on case-to-case basisb | - If lumbar puncture is performed, we recommend initiating treatment with IV acyclovir and following the sequence described above - If lumbar puncture is not performed, oral acyclovir may be considered as an alternative to intravenous treatment, and given at doses of 20mg/kg every 6h for 5–7 days |
NMDAr, N-methyl-d-aspartate receptor; IV, intravenous; CNS, central nervous system; CSF, cerebrospinal fluid; HSV, herpes simplex virus; PCR, polymerase chain reaction.
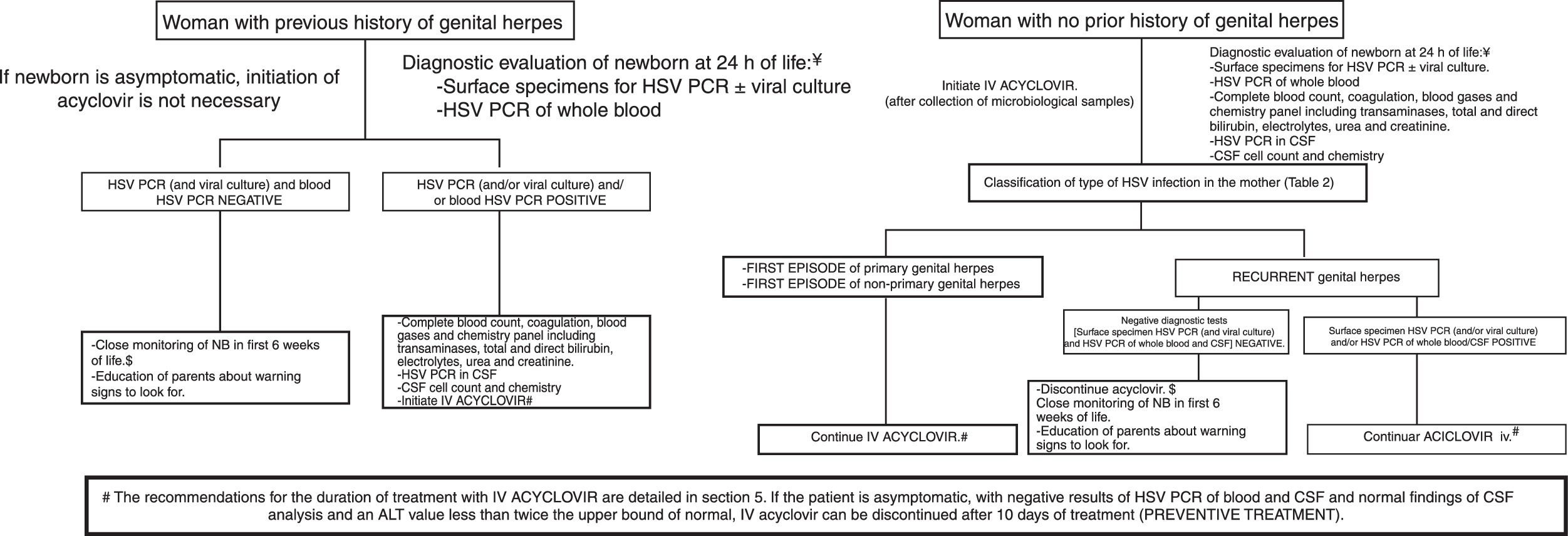
Fig. 1 describes the possible scenarios and the protocol for the management of children born to mothers with genital lesions associated with HSV infection at the time of delivery.34–36
Management of asymptomatic newborns delivered vaginally or by caesarean section and with mothers with genital lesions at the time of delivery. Before implementing this algorithm, the clinician should verify in collaboration with the microbiologist that the necessary techniques are available and the turnaround times are adequate.
¥The diagnostic evaluation and treatment will be performed earlier if the newborn presents signs of infection by HSV. Some experts recommend collection of specimens and initiation of treatment immediately after delivery in case of prolonged rupture of membranes or preterm birth.
$If the newborn is asymptomatic and the results of PCR negative, discharge at 48h post birth can be considered if the necessary conditions are met for adequate monitoring at home and immediate access to the hospital.
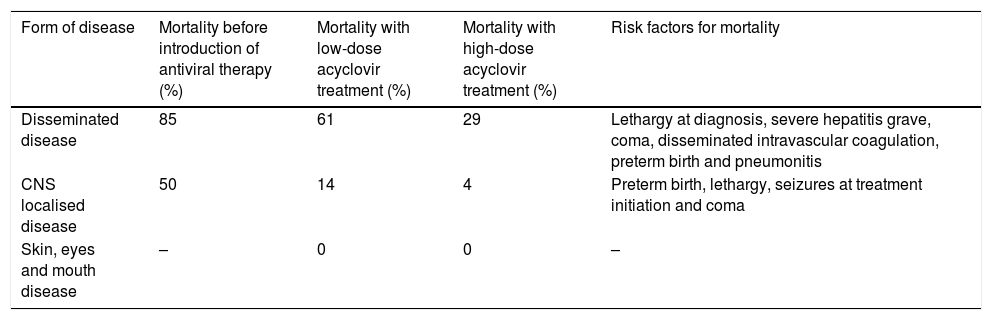
The mortality rate associated with NH is of approximately 0.8 deaths per 100000 live births.37 Mortality has declined significantly after the introduction of antiviral treatment (Table 6).21,29,35 Antiviral treatment has not only succeeded in preventing the progression of cutaneous disease, owing to which neurologic outcomes in these patients are currently excellent, but is also associated with significantly improved neurologic outcomes in cases of disseminated disease. The introduction of suppressive therapy has also been a substantial step forward, improving neurologic outcomes in patients with CNS involvement.22,24,33,35,38
Mortality at age 1 year by form of neonatal infection by HSV.22,33,40
| Form of disease | Mortality before introduction of antiviral therapy (%) | Mortality with low-dose acyclovir treatment (%) | Mortality with high-dose acyclovir treatment (%) | Risk factors for mortality |
|---|---|---|---|---|
| Disseminated disease | 85 | 61 | 29 | Lethargy at diagnosis, severe hepatitis grave, coma, disseminated intravascular coagulation, preterm birth and pneumonitis |
| CNS localised disease | 50 | 14 | 4 | Preterm birth, lethargy, seizures at treatment initiation and coma |
| Skin, eyes and mouth disease | – | 0 | 0 | – |
CNS, central nervous system; HSV, herpes simplex virus.
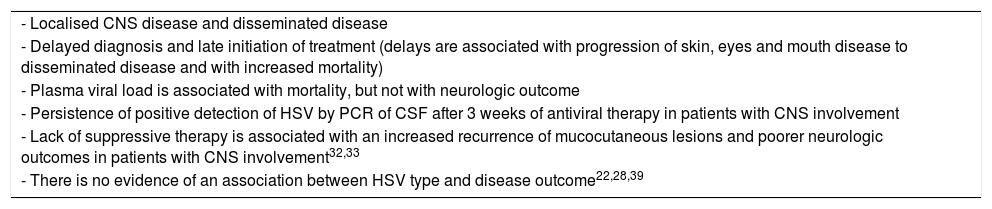
Table 7 presents the prognostic factors for NH.
Prognostic factors that predict a poor outcome of neonatal infection by HSV.
| - Localised CNS disease and disseminated disease |
| - Delayed diagnosis and late initiation of treatment (delays are associated with progression of skin, eyes and mouth disease to disseminated disease and with increased mortality) |
| - Plasma viral load is associated with mortality, but not with neurologic outcome |
| - Persistence of positive detection of HSV by PCR of CSF after 3 weeks of antiviral therapy in patients with CNS involvement |
| - Lack of suppressive therapy is associated with an increased recurrence of mucocutaneous lesions and poorer neurologic outcomes in patients with CNS involvement32,33 |
| - There is no evidence of an association between HSV type and disease outcome22,28,39 |
CNS, central nervous system; CSF, cerebrospinal fluid; HSV, herpes simplex virus; PCR, polymerase chain reaction.
The main sequelae are neurologic impairment (psychomotor retardation, microcephaly, paresis, spasticity, epilepsy, learning disabilities), ocular impairment (cortical blindness, corneal and chorioretinal scars, optical atrophy, cataracts, acute retinal necrosis and oculomotor disorders) and hearing impairment (neurosensory hearing loss).
We recommend that the followup of patients with NH be performed by a multidisciplinary team.33,35
Recent evidence has emerged that in cases of HSV infection with CNS involvement, the infection can trigger autoimmune encephalopathy, in most cases with presence of anti-N-methyl-d-aspartate receptor antibodies (anti-NMDAr Abs). Up to 20% of relapses in patients with CNS infection by HSV may be immune-mediated.40 The encephalopathy usually develops weeks or months after the acute infection by HSV, and the most frequent feature in young children is choreoathetosis, with other frequent manifestations including decreased level of consciousness and convulsive seizures.40 Patients with this clinical presentation should be assessed with determination of neuronal cell-surface antibodies in serum and CSF (mainly anti-NMDAr Abs). Some authors recommend starting empiric immunotherapy in patients with these symptoms and negative results of CSF PCR for HSV while awaiting the antibody results.40
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank David Tarragó Asensio, for the critical review of the manuscript and his valuable contributions.
Coordinators (in alphabetical order)
Writers (in alphabetical order)
Appendix A lists the names of all the authors of the article.
Please cite this article as: Grupo de Trabajo de Infección Neonatal por virus herpes simplex de la Sociedad Española de Infectología Pediátrica. Guía de la Sociedad Española de Infectología Pediátrica sobre prevención, diagnóstico y tratamiento de la infección neonatal por virus herpes simplex. An Pediatr (Barc). 2018;89:64.