Children with primary immunodeficiency have severe life-threatening infections and a higher prevalence of autoimmune problems, allergy and lymphoproliferative disorders. Allogenic hematopoietic stem cell transplantation has been the only potentially curative option.
Patients and methodsPatients with primary immunodeficiency underwent allogenic stem cell transplantation in the period 1985–2011, and registered in the Spanish Working Party for Bone Marrow Transplantation in Children.
ResultsOne hundred and fifty-nine patients underwent 173 allogenic stem cell transplantations, of whom 97 had severe combined immunodeficiency, 30 with immune dysregulation disorders, 25 Wiskott-Aldrich syndrome, and 21 phagocyte disorders.
The median patient age at diagnosis was 6 months (range: 17 days–168 months) and the median patient age at transplant was 12 months (range: 1–189 months).
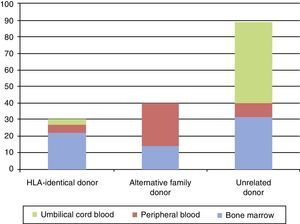
The donors were 30 (19%) identical siblings, 40 (25%) alternative family donors, and 89 (56%) unrelated donors. The source of stem cells was bone marrow in 68 (43%), cord blood in 52 (33%), and peripheral blood in 39 (24%).
Ninety-eight (61.6%) patients are alive and 57 (35.9%) died. Event-free survival at 10 years was 63%, with 90% for children transplanted from identical siblings, 36% for those transplanted from alternative family donors, and 66% for those transplanted from unrelated donors.
ConclusionsThe best results have been obtained with identical siblings, but other options may be considered.
Los niños afectados de inmunodeficiencias primarias presentan infecciones graves y mayor prevalencia de manifestaciones autoinmunitarias, alergias y enfermedad linfoproliferativa. El trasplante alogénico de precursores hematopoyéticos ha sido el único tratamiento curativo durante décadas.
Pacientes y métodosPacientes con inmunodeficiencias primarias que recibieron trasplante alogénico de precursores hematopoyéticos desde 1985 hasta 2011, recogidos en el Registro Nacional del Grupo Español para Trasplante de Médula Ósea en Niños.
ResultadosCiento cincuenta y nueve niños recibieron un total de 173 trasplantes, 97 por inmunodeficiencia combinada grave, 30 por enfermedades de disregulación inmunitaria, 25 por síndrome de Wiskott-Aldrich y 21 por defectos de número y/o función de los fagocitos.
La mediana de edad al diagnóstico fue de 6 meses (17 días-168 meses) y de 12 meses (1 mes-189 meses) al trasplante.
Los donantes fueron hermano HLA idéntico en 30 (19%), donante familiar alternativo en 40 (25%) y donante no emparentado en 89 (56%). La fuente de progenitores fue médula ósea en 68 (43%), sangre de cordón umbilical en 52 (33%) y sangre periférica en 39 (24%).
Permanecen vivos 98 niños (61,6%), 57 (35,9%) fallecieron. La supervivencia libre de enfermedad a los 10 años fue del 63, el 90% para los pacientes trasplantados de hermano HLA idéntico, el 36% para los trasplantados de un donante familiar alternativo y el 66 para los trasplantados de donante no emparentado.
ConclusionesLos mejores resultados se obtienen con un hermano HLA idéntico, cuando no se dispone de este, otros donantes deben ser considerados.
Primary immunodeficiencies (PIDs) are a heterogeneous group of over 200 congenital diseases caused by quantitative or functional defects of different mechanisms involved in the immune response. They are characterised by a poor response to infectious agents, leading to recurrent infection and a greater prevalence of autoimmune manifestations, allergies, and lymphoproliferative disorders.1
Around 60% of these diseases are diagnosed during childhood.1
The current classification of the International Union of Immunology Societies defines 8 groups of PIDs: combined T- and B-cell immunodeficiencies; predominantly antibody deficiencies; diseases of immune dysregulation; defects of phagocyte number, function, or both; defects in innate immunity; complement disorders; autoinflammatory disorders; and other well-defined immunodeficiency syndromes.1,2
The clinical manifestations of PIDs are broad in scope, and the following are the main warning signs that an immunodeficiency should be suspected: recurrent infections (more than 8 new episodes of otitis media within a year; more than 2 pneumonias confirmed radiologically within a year; more than 2 episodes of sinusitis within a year; more than 2 deep-tissue infections or infections in unusual locations within a year; recurrent deep skin infections or abscesses; infection by unusual or opportunistic organisms; 2 or more episodes of meningitis or severe infection); failure to gain weight or grow normally; recurrent autoimmune phenomena; thrush in mouth or fungal infection of the skin in patients older than one year; dysmorphic features related to frequent infections; infection after vaccination with live virus vaccines; delayed umbilical cord separation; recurrent mouth sores; fever with suspected periodicity; and unexplained bronchiectases.
Severe combined immunodeficiency (SCID) is the most severe form of PID, with a life expectancy of less than a year in the absence of an allogeneic hematopoietic stem cell transplantation (allo-HSCT) or the genetic correction of the underlying defect.3 In other PIDs, such as defects in innate immunity or other T-cell immunodeficiencies, life expectancy does not exceed three or four decades.4
For decades, allo-HSCT was the only curative treatment for a broad range of PIDs in children.4–6 In 1968, the first HLA-identical sibling bone marrow transplantation was performed successfully in a child with severe combined immunodeficiency.7 Since then, transplants from other sources and types of donor have been used with good results.4–6 Haploidentical donor and umbilical cord blood (UCB) transplants are two valid options that can be used in cases in which allo-HSCT is a medical emergency.4–6,8,9
Immune reconstitution following allo-HSCT is a slow process, and normal counts of circulating T-cells are not reached until 6–12 months after the transplant, so morbidity and mortality can be high in the first year post-transplantation.8
We present the outcomes of allo-HSCTs in children with PIDs documented in the registry of the Grupo Español para el Trasplante de Médula Ósea en Niños (Spanish Working Group on Bone Marrow Transplants in Children [GETMON]).
Patients and methodsThis study included patients with PID that required an allo-HSCT between 1985 and 2011 registered in the GETMON national registry.
GETMON is a collaborative group formed by 14 Spanish hospitals that perform allo-HSCTs. It was formed with the purpose of developing shared protocols, assessing current practices, and expanding knowledge. With that objective in mind, a national registry was created to collect the basic data of all patients receiving an HSCT starting in 1985, for the purposes of epidemiological analysis. Analysing the data in this registry allows drawing conclusions on the indications of HSCT and on its outcomes.
The registry collects data on characteristics like age, sex, hospital, underlying disease, type of donor, type of hematopoietic stem cell source, and post-HSCT follow-up.
The data were collected after obtaining the informed consent of the parents or guardians.
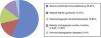
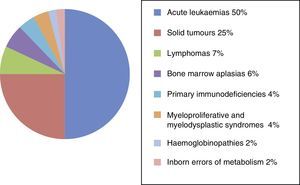
ResultsSince its founding in 1985, the National Registry of the GETMON has collected data on 4330 transplants performed on patients with acute leukaemia (50%), solid tumours (25%), lymphoma (7%), bone marrow aplasia (6%), primary immunodeficiencies (4%), myeloproliferative and myelodysplastic syndromes (4%), haemoglobinopathies (2%) and inborn errors of metabolism (2%) (Fig. 1).
Between February 1985 and December 2011, 159 patients with PIDs received a total of 173 allo-HSCTs in centres belonging to the GETMON. Four centres performed 156 (90%) of the 173 allo-HSCTs: 71 (41%) were done at the Hospital Vall d’Hebron, 41 (24%) at the Hospital La Paz, 29 (16%) at the Hospital del Niño Jesús, and 15 (9%) at the Hospital Santa Creu i Sant Pau. Fifty-five patients received an allo-HSCT between 1985 and 2001, and 104 patients received one between 2002 and 2011.
Patient characteristicsOf all patients, 109 (68%) were boys and 50 (32%) girls. The mean age at diagnosis was 6 months (ranges, 17 days–168 months).
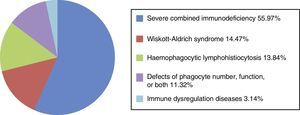
Ninety-seven patients were diagnosed with SCID (55.97%); 30 with immune dysregulation (17.34%; of which 24 [13.87%] had haemophagocytic lymphohistiocytosis; 6 [3.47%] had other unspecified immune dysregulation disorders); 25 (14.47%) with Wiskott-Aldrich syndrome (WAS); 21 with defects of phagocyte number and/or function (11.32%; congenital neutropaenia and chronic granulomatous disease) (Fig. 2).
Transplantation characteristicsThe total number of patients that received a transplant was 145; 14 of them required two. The characteristics of the first transplants (there were a total of 159) are described immediately below.
The donor was an HLA-identical sibling in 30 transplantations (19%), an alternative family donor (including haploidentical and single-antigen/allele mismatched donors) in 40 (25%) and an unrelated donor (URD) in 89 (56%).
The mean age at transplantation was 12 months (range, 1–189 months).
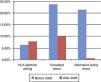
The source of hematopoietic stem cells was the bone marrow in 68 cases (43%), umbilical cord blood in 52 (33%), and peripheral blood in 39 (24%). The most common source for the HSCT was bone marrow in cases in which the donor was an HLA-identical sibling; peripheral blood for alternative family donors; and umbilical cord blood for URDs (Fig. 3).
Decisions concerning pre-transplant conditioning regimen, prophylactic treatment against graft-versus-host disease, and supportive care were made based on the underlying disease, the type of donor and source of transplant, and the protocols that were in place at each centre at different times.
Post-transplantation assessmentNinety-eight children (61.6%) survived; 57 (35.9%) died; and 4 (2.5%) were lost to follow-up.
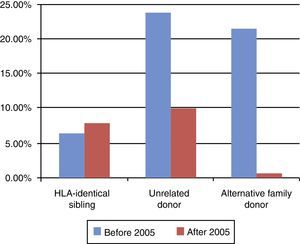
Mortality at 100 days post-transplantation varied depending on the type of donor, and decreased as years went by. It was lower in patients with transplants from an HLA-identical sibling than in patients with transplants from an alternative family donor or an URD (Fig. 4).
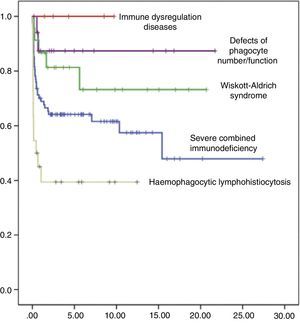
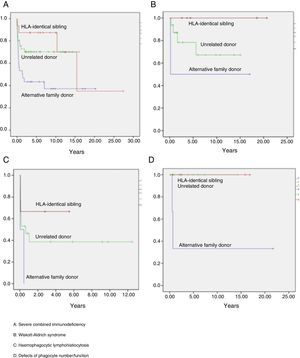
Event-free survival (EFS) at 10 years post-transplant was 63%, reaching 58% in the group of children who received transplants before year 2005, and 75% in children whose transplants were performed at a later date. In terms of the type of donor, EFS at 10 years post-transplant was 90% for transplants from an HLA-identical sibling, 60% for transplants from an URD, and 36% for transplants from an alternative family donor. When it came to EFS at 10 years post-transplant as a function of the type of primary immunodeficiency, it was 60% in children with SCID, 75% in children with WAS, 88% in children with phagocytic defects, and 40% in children with haemophagocytic lymphohistiocytosis (Fig. 5). The EFS curves for patients as a function of the type of PID and the type of donor are presented in Fig. 6.
DiscussionHistorically, patients with PID were the first group of paediatric patients treated with allo-HSCT (d). The first HSCT in a patient diagnosed with a PID was performed successfully in 1968; it consisted of a transplant of bone marrow cells from an HLA-identical sibling in a child with SCID.7 Since then, HSCT has become the curative treatment for more than 30 PIDs, with increasingly satisfactory outcomes.4–6
Patients with SCIDs have the best reported outcomes for allo-HSCTs of all patients with PIDs. SCIDs constitute the most severe form of PID, and are characterised by the absence of T cell-mediated immunity in all of them, and varying defects in B cell function.10,11 The phenotypic spectrum is comprised of T−B+ Nk+, T−B+ Nk−, T−B−Nk+, and T−B−Nk−. Patients with a T−B− SCID and with ADA deficiency carry a poorer prognosis.11
SCID is a medical emergency, and its early diagnosis and treatment can improve survival. To facilitate early diagnosis of this disease, several countries have implemented neonatal screening programmes based on PCR amplification, and in tandem mass spectrometry for ADA deficiency.12,13 Survival rates are better in patients who receive a transplant early, before 6 months of age.11,13 The transplant of choice in these patients is bone marrow from an HLA-identical sibling.11,14 The data published by the European SCETIDE/EBMT registry (1968–2005), which includes 699 patients with SCID that underwent an allo-HSCT, show a survival for the period ranging from 2000 to 2005 of nearly 90% in 25 patients who received a transplant from an HLA-identical sibling, of 66% in 96 patients who received a transplant from an alternative family donor, and of 69% in 46 with transplants from URDs.4 If the transplant is a medical emergency, both haploidentical and HSCT from UCB are valid options, with survival rates nearing 70%.3,14 The data in our series are consistent with this, with a survival of 88% in patients who received an allo-HSCT from an HLA-identical sibling and of 72% in patients who received transplants from URDs. The survival of patients who underwent a haploidentical transplant was significantly lower at 36%, but these are patients who received the transplant in the late 1980s and early 1990s, and the outcomes have improved since.
In the group of patients with non-SCID PIDs, which includes a broad range of diseases, such as WAS, haemophagocytic lymphohistiocytosis, congenital neutropaenia, and chronic granulomatous disease, allo-HSCT is reserved for those that develop complications or severe infections.4 The transplant of choice in these patients is also the bone marrow of an HLA-identical sibling. The data published by the SCETIDE/EBMT European registry (1968–2005), which includes 783 patients of non-SCID PID that underwent an allo-HSCT, show a survival for the period ranging from 2000 to 2005 that nears 80% in 73 patients with transplants from an HLA-identical sibling.4 Survival for transplants from URDs (124 cases) and alternative family donors (23 cases) ranged between 45% and 65% depending on HLA matching.4 The overall survival of this group of patients has increased in recent years, although it still does not reach the rates achieved by the SCID group. Our series confirms this trend, with, for instance, a survival of 100% for HSCTs from HLA-identical siblings, of 65% in HSCTs from URDs, and 55% in HSCTs from alternative family donors in patients with WAS.
The conditioning for all types of PID involved myeloablative or reduced-intensity regimens depending on the type of donor, the source of hematopoietic stem cells used and the associated comorbidities.8,15 In recent years, better outcomes have been observed in patients conditioned with reduced-intensity regimens, especially in patients with non-SCID PIDs.15 And in certain circumstances, such as patients with SCID receiving a transplant from an HLA-identical sibling, administration of conditioning treatment may even be unnecessary.11
The reconstitution of normal levels of circulating T lymphocytes does not happen until 6–12 months after the HSCT, which may result in high morbidity and mortality.8 Some patients may never reach normal B cell counts, and they will remain dependent on gamma globulin transfusions.8
At present, HSCT remains the treatment of choice for a broad range of PIDs. The first cases of patients treated with gene therapy have been published, and while some complications associated with this approach have been reported, such as development of leukaemias following gene therapy for X-linked SCID, potential solutions for these problems are currently being investigated.16–19
Despite its limitations, this retrospective descriptive study allows us to draw conclusions regarding the outcomes of allo-HSCT in patients with PID in Spain, and to compare the results obtained with results published at the international level.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Hladun R, Badell I, González M, Martínez AM, Sánchez de Toledo J, Olivé MT, et al. Análisis de la supervivencia de los niños con inmunodeficiencias primarias que han recibido un trasplante de progenitores hematopoyéticos en España. Anales de Pediatría. 2015;82:62–67.