Intentional drug poisoning with suicidal intent is an important cause of morbidity and mortality in adolescents.1
The drugs involved most frequently in these cases are paracetamol, ibuprofen and, third in frequency, selective serotonin reuptake inhibitors (SSRIs).2,3 The frequency of the latter has been increasing in recent decades because these drugs are used for first-line treatment of major depression and anxiety disorders in this age group.4
The aim of our study was to assess the clinical manifestations, laboratory findings and electrocardiographic features associated with intentional ingestion of SSRIs with suicidal intent in adolescents.
We conducted a single-centre retrospective observational study in patients aged less than 18 years managed between February 2013 and May 2018 in the paediatric emergency department (PED) of a tertiary care hospital that is a reference centre for psychiatric disorders with a discharge diagnosis of “intentional drug poisoning” or “suicide attempt”. We selected patients in who the main drug ingested in the episode was a SSRI (Table 1). We defined intentional drug poisoning as ingestion of a drug in amounts exceeding the maximum dose established in the marketing authorisation with suicidal intent. The statistical analysis consisted of parametric tests performed with the software Stata version 15.
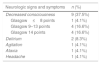
Type of SSRI used.
| SSRI | n (%) | Number of patients that ingested a dose exceeding the toxic thresholda |
|---|---|---|
| Fluoxetine | 14 (58.3%) | 13 |
| Sertraline | 4 (16.6%) | 2 |
| Escitalopram | 4 (16.6%) | 3 |
| Paroxetine | 1 (4%) | 0 |
| Trazodone | 1 (4%) | 1 |
SSRI, selective serotonin reuptake inhibitor.
In the period under study, the PED received 306 583 visits. Twenty-four patients were treated for intentional ingestion of SSRI, all of them female, with a median age of 15.7 years (interquartile range [IQR], 14.4–16.7). Twenty-three (95.8%) had an underlying mental health disorder. The median time elapsed from ingestion of the drug to assessment at the PED was 1.5h (IQR, 1–2), and 13 (54.2%) were deemed stable in the initial paediatric assessment triangle (PAT); 10 (41.7%) presented with neurologic impairment and 1 had compensated shock (4.1%). All patients with neurologic impairment had ingested not only SSRIs, but also benzodiazepine or antipsychotic drugs. The most frequent symptoms were neurologic (Table 2), followed by vomiting (4; 16.6%). The neurologic symptoms had resolved spontaneously by a median of 8h post ingestion (IQR, 6.5–18), and all patients maintained a patent airway and spontaneous breathing at all times without haemodynamic or respiratory abnormalities.
Sixteen (66.7%) patients underwent urgent gastrointestinal decontamination. We did not find clinically relevant abnormalities in any of these patients in the electrocardiogram or the serial laboratory tests performed (blood gases, kidney and liver enzymes, creatinine phosphokinase), so no additional treatment was required. All patients were admitted to hospital: 11 to the intensive care unit (ICU) for continuous monitoring, and 13 in the inpatient ward, and none required respiratory or haemodynamic support. The median length of stay was 8 days (IQR, 4–20).
In conclusion, the patients that received care in our hospital for ingestion of SSRIs with suicidal intent did not exhibit any relevant electrocardiographic or blood test abnormalities. Larger studies are required to confirm these findings.
FundingThe authors declare not having received any external funding for this study.
Please cite this article as: Cuenca Carcelén S, Moral Larraz A, Sanchíz Perea A, Alonso Cadenas JA, de la Torre Espí M. Intento autolítico con antidepresivos inhibidores selectivos de la recaptación de serotonina. An Pediatr (Barc). 2021;94:51–52.