Among small for gestational age neonates, foetal Doppler enables the identification of placental insufficiency aetiology and the classification of severity in small for gestational age neonates. There are studies that associate the Doppler data with alterations in the intestinal flow of the newborn, but its relationship with intestinal oximetry has been little studied.
ObjectiveTo assess whether there is a relationship between prenatal Doppler data and abdominal oximetry in small for gestational age neonates.
Material and methodsA prospective observational study carried out on neonates >32 weeks with a birth weight
ResultsA total of 53 patients were evaluated. Significant differences were observed in the mean regional oximetry (rSO2) between patients with moderate or severe placental failure and those with normal or slightly altered prenatal Doppler: 42±10 vs. 71.3±10 (P<.001). These differences were maintained during the first 3 days of life. Standard patterns of splanchnic oximetry were identified depending on the degree of placental insufficiency.
ConclusionsThere is a correlation between the foetal Doppler and the splanchnic oximetry pattern during the first days of life. Neonates with moderate or severe placental insufficiency have more altered abdominal oximetry patterns, making it a useful technique to evaluate the degree of placental insufficiency and the risk of oral intolerance in small for gestational age neonates.
El estudio del doppler foetal permite identificar la etiología placentaria y clasificar su gravedad en aquellos neonatos pequeños para la edad gestacional. Existen estudios que relacionan estos datos doppler con alteraciones en el flujo intestinal del recién nacido, pero su relación con los datos de oximetría intestinal ha sido poco estudiada.
ObjetivoEvaluar si existe relación entre los datos doppler prenatales y los datos de oximetría abdominal en los niños pequeños para su edad gestacional.
Material y métodosEstudio prospectivo observacional en neonatos>32 semanas con un peso al nacer<P10. Se clasificó la gravedad de la insuficiencia placentaria según criterios de doppler prenatal. Se monitorizó la oximetría esplácnica durante los 3 primeros días de vida y se realizó un análisis comparativo de los datos oximétricos según la afectación doppler prenatal.
ResultadosSe evaluaron 53 pacientes. Se observaron diferencias significativas en la rSO2 media entre los pacientes con fallo placentario moderado o grave y aquellos con doppler prenatal normal o levemente alterado: 42±10 vs. 71,3±10 (p<0,001). Estas diferencias se mantuvieron durante los 3 primeros días de vida. Se identificaron patrones tipo de oximetría esplácnica según el grado de insuficiencia placentaria.
ConclusionesExiste correlación entre el doppler foetal y el patrón de oximetría esplácnica durante los primeros días de vida. Los neonatos con insuficiencia placentaria moderada o grave presentan unos registros de oximetría abdominal más alterados, por lo que podría ser una técnica útil para evaluar el grado de insuficiencia placentaria y el riesgo de intolerancia oral en los neonatos pequeños para la edad gestacional.
Deficient intestinal perfusion is one of the factors that contribute to the development of gastrointestinal disease in infants,1 so in recent years various strategies have been developed to assess blood flow in the splanchnic vascular bed in at-risk newborns. Initial assessments were based on the examination of the superior mesenteric artery by Doppler ultrasound,2 but its applicability in clinical practice was poor since this is a complicated technique that does not allow continuous monitoring and requires an experienced sonographer for its implementation.3–7
Near-infrared spectroscopy (NIRS) tissue oximetry is another technique recently introduced in neonatal units. This method allows the continuous and non-invasive monitoring of tissue oxygen saturation.8 There is evidence that the data obtained using this method in newborns offer accurate information on the oxygenation of the tissue on which the sensor is placed.9,10 This method was initially used to measure regional cerebral oxygen saturation in children undergoing surgery with extracorporeal circulation, and its use was later expanded to other groups of patients at neurologic risk.11 Studies have also been conducted in the field of neonatology regarding the usefulness of NIRS for monitoring of cerebral oxygen saturation in preterm infants.12–17 Studies of regional oxygen saturation at the level of the bowel (splanchnic oximetry) started being published more recently and have smaller sample sizes, but have demonstrated the potential of this technique for monitoring intestinal perfusion in patients at risk of intestinal ischaemia.18,19
Newborns that are small for gestational age (SGA) have specific characteristics that, as is the case of preterm newborns or those with congenital heart defects, may lead to intestinal perfusion problems. In recent years, there have been significant advances in the diagnosis and management of SGA foetuses and infants, moving from a merely statistical construct based on having a birth weight below the 10th percentile for gestational age to a pathophysiological conceptualization that takes into account the findings of prenatal Doppler ultrasound examinations and differentiates between infants with low birth weight (LBW) for gestational age, either constitutional or associated with foetal or maternal disease, and infants with a history of abnormal findings in prenatal Doppler ultrasound examinations, which signal the presence of intrauterine growth restriction (IUGR).
The primary objective of our study was to assess the splanchnic oximetry values recorded in SGA newborns relative to the prenatal Doppler ultrasound data. The secondary objectives were to assess the association of splanchnic oximetry values with the severity revealed by Doppler ultrasound data, to search and describe oximetry patterns associated with the degree of severity of placental insufficiency and to assess the association between splanchnic oximetry values and oral feeding tolerance in the infants.
Material and methodsWe carried out a prospective observational study in a level III-C neonatal unit over a 20-month period (April 2017–December 2018).
We included all infants born at 32 weeks’ gestation or later admitted to the neonatal unit with a birth weight below the 10th percentile in the 2008 Carrascosa tables20,21 and whose parents or legal guardians signed the informed consent form. We excluded newborns with congenital malformations that required advanced life support or required mechanical ventilation (invasive or non-invasive) with a fraction of inspired oxygen (FiO2) of more than 30% for 12 or more hours.
We recorded all the data in a data collection notebook. We collected data on demographic characteristics, baseline clinical variables and postnatal outcomes variables from the health records of the patients. We initiated monitoring by splanchnic oximetry within 6h of birth with the INVOS™ 5100-C oximeter (Somanetics, Medtronic, MN, USA) and a neonatal sensor placed in the left abdomen at the level of the navel or below the navel. We monitored splanchnic blood flow continuously for 3 days, documented the mean value and range of the regional oxygen saturation (rSO2), values of rSO2 below 30% and the oximetry pattern every 24h using the software INVOS™ Analytics Tool version 1.2.
The definition of SGA has been under debate in recent years, as the classification as such of infants with a birth weight below the 10th percentile does not take into consideration prenatal Doppler ultrasound data.22,23 In our study, we applied the definition of SGA used in our perinatal care area, which is being adopted by an increasing number of units in Spain that differentiates infants with LBW from infants with a history of IUGR defined as abnormalities in the prenatal Doppler ultrasound.24
To stage intrauterine growth restriction, we used the classification of Figueras and Gratacós of 2017.24 Thus, we differentiated between low weight for gestational age (constitutional or secondary) and IUGR (due to placental insufficiency). The latter was further subdivided into 4 stages (I, II, III and IV), each of which indicates increasing severity, based on the data obtained from the Doppler ultrasound examination of the umbilical artery, middle cerebral artery and ductus venosus of the foetus.
Stage I IUGR, the mildest, is defined by the presence of Doppler features defining IUGR in the absence of signs of progression (abnormal uterine artery Doppler findings or detection of involvement of umbilical artery on Doppler ultrasound). Stage II is defined by foetal blood flow redistribution evinced by Doppler ultrasound examination of the foetal middle cerebral artery with features suggestive of progression with involvement of the uterine artery (moderate IUGR). Stage III involves greater severity and is defined by the presence of the features defining previous stages combined with cardiac involvement with abnormalities in the ductus venosus (severe IUGR). Stage IV is defined by increased involvement of the ductus venosus and signals a high risk of severe hypoxia and foetal acidosis and is an indication for urgent caesarean delivery. None of the patients in our sample had stage IV IUGR.
The nutrition protocol applied during the study was the same in all patients. Feeding intolerance was defined as the need to keep the patient nil per os for more than 12 consecutive hours.
We performed a descriptive analysis of all the variables, expressing quantitative data as mean±standard deviation and median, and qualitative data as absolute frequencies and percentages. We compared qualitative variables in the 2 groups using the chi square test, and means with the Student t test or the Mann–Whitney U test as applicable based on the assessment of normality with the Kolmogorov-Smirnov test. We assessed the association between quantitative variables by means of the Spearman correlation coefficient. Statistical significance was defined as a P-value of less than 0.05 (two-tailed). We performed a multivariate multiple linear regression analysis adjusted for gestational age. All the analyses were performed using the software SPSS® version 24.0.
Before starting the study, we obtained the approval of the competent Research Ethics Committee.
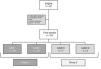
ResultsThe sample included a total of 53 patients. Fig. 1 provides a flow chart that details the recruitment process.
We did not find significant differences in rSO2 values between LBW patients compared to patients with stage I IUGR (95% CI, −0.5–12; P=.066). For this reason, in subsequent analyses we grouped these 2 sets of patients into a single group, Group 1, for comparison with patients with a history of high-grade placental insufficiency (IUGR stages II--III), who constituted Group 2.
Table 1 presents the data for the total sample and the bivariate analysis comparing Groups 1 and 2 in terms of baseline, perinatal and postnatal outcome variables. Table 2 presents the results of the bivariate analysis of the two groups comparing splanchnic oximetry variables for each of the 3 days of monitoring.
Data for the overall sample and the bivariate analysis.
| Totaln=53 | Group 1n=44 (83%) | Group 2n=9 (16%) | P | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Male, n (%) | 23 (43.4%) | 17 (38.6%) | 6 (66.7%) | NS |
| Gestational age (days) | ||||
| Mean±SD | 251.9±13.6 | 255±11.8 | 236±11.1 | <.001 |
| Median (IQR) | 258 (241.5–261) | 259 (250–262) | 237 (224–244) | |
| Preeclampsia, n (%) | 16 (30.2%) | 13 (29.5%) | 3 (33.3%) | NS |
| Twin pregnancy, n (%) | 5 (9.4%) | 3 (6.8%) | 2 (22.2%) | NS |
| Gestational diabetes, n (%) | 5 (9.4%) | 4 (9.1%) | 1 (11.1%) | NS |
| Uncomplicated birth, n (%) | 17 (32.1%) | 5 (11.4%) | 2 (22.2%) | NS |
| CTG abnormalities n (%) | 14 (26.4%) | 12 (27.3%) | 2 | NS |
| Apgar 1 | ||||
| Mean±SD | 8±1 | 8 (5–9) | 7 (6–9) | NS |
| Median (IQR) | 9 (7–9) | 9 (7–9) | 7 (6.5–8) | |
| Apgar 5 | ||||
| Mean±SD | 9±1 | 9 (8–10) | 9 (8–10) | NS |
| Median (IQR) | 9 (7–9) | 10 (9–10) | 9 (8–9.5) | |
| pH at admission | ||||
| Mean±SD | 7.31±0.07 | 7.32±0.07 | 7.27±0.09 | NS |
| Median (IQR) | 7.33 (7.28–7.36) | 7.34 (7.29–7.36) | 7.27 (7.19–7.35) | |
| Lactate | ||||
| Mean±SD | 4.1±2.5 | 4.35±2.64 | 3.02±1.36 | NS |
| Median (IQR) | 3.4 (2.7–4.7) | 3.4 (2.8–5.5) | 3.1 (1.9–4.4) | |
| Haematocrit | ||||
| Mean±SD | 54.1±6.3 | 54.6±6 | 51.9±7 | NS |
| Median (IQR) | 54.6 (49.9–58.9) | 54.7 (50.4–59) | 53 (44.7–58.6) | |
| Birth weight | ||||
| Mean±SD | 1.819±329 | 1902±285 | 1415±209 | <.001 |
| Median (IQR) | 1.900 (1.545–2.090) | 1.985 (1.665–2.107) | 1.440 (1.252–1.585) | |
| Birth weight percentile | ||||
| Mean±SD | 2.5±2.1 | 2.6±2.1 | 2.1±2 | NS |
| Median (IQR) | 2 (1–3) | 2 (1–3) | 1 (1–3) | |
| Birth length | ||||
| Mean±SD | 43.3±2.4 | 43.7±2.2 | 41.3±2.3 | <.001 |
| Median (IQR) | 44 (41.5–45.3) | 44 (42–45.5) | 42 (39–43) | |
| Birth length percentile | ||||
| Mean±SD | 7.7±11 | 6.5±7.5 | 13.9±21.0 | NS |
| Median (IQR) | 3 (1–11.5) | 2 (1–12.25) | 5 (2–25) | |
| Head circumference | ||||
| Mean±SD | 30.4±1.8 | 30.9±1.5 | 27.8±1.4 | <.001 |
| Median (IQR) | 31 (29–31.7) | 31 (30–32) | 28 (27–28.7) | |
| Head circumference percentile | ||||
| Mean±SD | 7.6±11.4 | 8.25±12.1 | 4.44±6.2 | NS |
| Median (IQR) | 4 (1–8.5) | 4 (1–8.75) | 1 (1–8) | |
| Nutrition-gastrointestinal | ||||
| Time to full enteral feeding | ||||
| Mean±SD | 5.6±2.4 | 4.95±0.8 | 8.4±4.5 | <.001 |
| Median (IQR) | 5 (4–6) | 5 (4–5) | 7 (5.5–12) | |
| Delayed passage of meconium, hours | ||||
| Mean±SD | 14.7±11.1 | 14.5±10.5 | 16.1±14.4 | NS |
| Median (IQR) | 12.5 (7–19.7) | 13 (7–19) | 10 (3–30) | |
| Feeding intolerance, n (%) | 3 (5.7%) | 0 (0%) | 3 (33.3%) | <.001 |
| NEC, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | NS |
CTG, cardiotocography; IQR, interquartile range; NEC, necrotising enterocolitis; NS, not significant; SD, standard deviation.
Splanchnic oximetry value in the 3 days of monitoring.
| Totaln=53 | Group 1n=44 (83%) | Group 2n=9 (16%) | P | |
|---|---|---|---|---|
| rSO2(day 1) | ||||
| Mean±SD | 66.3±14.8 | 71.3±10 | 42±10 | <.001 |
| Median (IQR) | 68 (57.5–75) | 71.5 (65.3–79.5) | 44 (32–47.5) | |
| rSO2(day 2) | ||||
| Mean±SD | 66.9±14.8 | 70.7±12 | 48±12 | <.001 |
| Median (IQR) | 69 (56.5–77.5) | 72 (63.3–79.7) | 50 (37–57) | |
| rSO2(day 3) | ||||
| Mean±SD | 69.5±16.1 | 74.3±12.2 | 47.6±14.1 | <.001 |
| Median (IQR) | 74 (61–81) | 76 (67.7–82.3) | 53 (34–59) | |
| Values <30% (day 1) | ||||
| Mean±SD | 8±14 | 2.7±4.8 | 34.5±15.2 | <.001 |
| Median (IQR) | 1.2 (0–7.8) | 1 (0–3.6) | 28 (22.9–51.9) | |
| Values <30% (day 2) | ||||
| Mean±SD | 6.7±12.7 | 3.7±7.4 | 21.3±21.5 | <.001 |
| Median (IQR) | 1 (0–9) | 1 (0–2.3) | 13.5 (6.4–33.3) | |
| Values <30% (day 3) | ||||
| Mean±SD | 6.9±16 | 3.0±8.3 | 24.8±28.5 | <.001 |
| Median (IQR) | 1.6 (0–5.3) | 0.9 (0–2.1) | 11.3 (5–49.5) | |
| Pattern 1 (day 1), n (%) | 44 (83%) | 43 (97.7%) | 1 (11.1%) | <.001 |
| Pattern 1 (day 2), n (%) | 44 (83%) | 40 (90.9%) | 4 (44.4%) | <.001 |
| Pattern 1 (day 3), n (%) | 49 (92.5%) | 43 (97.7%) | 6 (66.7%) | <.001 |
IQR, interquartile range; rSO2: regional oxygen saturation; SD, standard deviation.
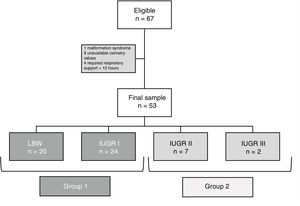
We found higher oxygen saturation values in Group 1 compared to Group 2, as can be seen in Fig. 2, which offers a graphic representation of the mean regional abdominal oxygen saturation values in the first 3 days post birth in both groups.
We performed a multiple linear regression analysis comparing patients with LBW, IUGR stage I and IUGR stages II–III corrected for gestational age and found no differences in oximetry values based on gestational age, but we did find differences associated with the prenatal Doppler findings in patients with high-grade placental insufficiency. The regression analysis showed that oximetry values in infants with high-grade IUGR had a mean rSO2 on day 1 that was 23 percentage points lower (95% CI, −13% to −33%; P<.001) compared to infants with stage I IUGR or LBW, independently of gestational age.
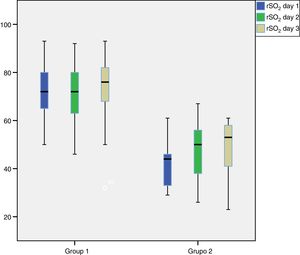
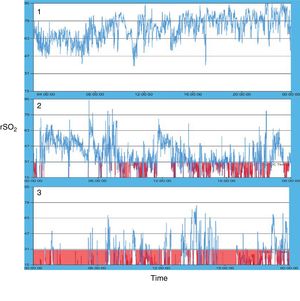
Fig. 3 presents the 3 characteristic oximetry patterns found in our series. Pattern 1 was associated with patients in the LBW or mild IUGR groups (Group 1), while patterns 2 and 3 were observed in patients with high-grade placental insufficiency (Group 2). Within Group 2, we only observed pattern 3 in patients with the highest grade of IUGR (stage III).
We found a correlation between the mean abdominal rSO2 and the days elapsed until full enteral feeding was achieved (correlation coefficient for rSO2 on day 1=0.58; P<.001). Patients in Group 2 exhibited poorer oral tolerance, defined as a need of nil per os lasting longer than 12 consecutive hours, compared to Group 1, a difference that was statistically significant. None of the patients in Group 1 had feeding intolerance, while its frequency in Group 2 was of 33.3% (3/9) (P<.001). None of the patients in our sample developed necrotising enterocolitis.
DiscussionPrevious studies have demonstrated the association between abnormal Doppler findings in the foetal umbilical artery and the superior mesenteric artery of the newborn,25,26 or the association with oral tolerance in newborns,26,27 but the association with splanchnic oximetry values had not been previously described save for a few small studies that did not fully assess SGA infants.
In our study, we found that oximetry values in patients with the mildest grade of placental insufficiency, or stage I IUGR, who constituted the majority of patients SGA due to placenta-related causes, did not differ from the values in LBW infants. We also found no differences between these groups in terms of oral tolerance. However, we found poorer oximetry values in patients with greater involvement of the umbilical artery on Doppler ultrasound, who had lower mean oxygen saturations at the splanchnic level. Oximetry values in newborns were poorer the more severe the abnormality detected in the prenatal Doppler ultrasound. Thus, patients with the most severe grade of placental insufficiency (stage III IUGR) had the poorest splanchnic saturation values and the longest duration of abnormal values. Patients with stage III IUGR were also the patients that exhibited feeding intolerance.
In foetuses with more severe IUGR, the Doppler ultrasound detects signs of adaptation to the moderate or severe hypoxia they experience due to the progression of placental insufficiency, with redistribution of centralization of blood flow. This redistribution favours perfusion of the cerebral vascular bed, reducing blood flow in other organs, including the intestine. This could explain the presence of intestinal hypoxia at birth that may be evinced by oximetry monitoring in the early days of life.
As described by Cortez et al.28 the identification of patterns in rSO2 trends (mean values and variability) is the most useful approach to the interpretation of recordings in monitored patients. The oximetry patterns shown in Fig. 3 provide a graphic summary of the data found in our study. Pattern 1 was significantly associated with patients with LBW or mild IUGR and characterised by high mean rSO2 values (>50–55%) and an acceptable variability in the recordings (more than 20% points/hour). Patterns 2 and 3 were significantly associated with a history of severe placental insufficiency. Few patients in our case series had feeding intolerance, but it is worth noting that none of the patients with pattern 3 had good oral tolerance. The association between very low splanchnic oxygen saturation values and feeding intolerance or a high risk of necrotising enterocolitis has been described in several studies published in recent years.29–34
Our study had limited power in comparing feeding intolerance in the 2 defined groups. Some authors35 have observed that abnormal flow in the umbilical artery (absent or reversed end diastolic flow) correlate to lower oximetry values in patients that developed intestinal morbidity. Similar findings have been described in studies carried out in patients at high risk of intestinal hypoxia, such as patients with congenital heart disease36 or preterm infants with haemodynamically significant ductus arteriosus and secondary diastolic steal in the descending aorta.37–39 We believe that the incidence of feeding intolerance is greater in this group of patients due to hypoxia in the splanchnic region and that the increased intestinal morbidity observed in SGA infants is largely due to the intestinal ischaemia found in infants with a history of IUGR, especially those with more severe prenatal Doppler abnormalities.
In patients with a history of IUGR, splanchnic oximetry may be useful as an indicator of the severity of placental insufficiency experienced in utero, as it allows the assessment at birth of the degree of blood flow redistribution secondary to chronic hypoxia during foetal life. In addition, based on the findings of other studies that found an association between very low oxygen saturation values, feeding intolerance and an increased risk of necrotising enterocolitis,29,40 splanchnic oximetry monitoring could be useful to determine the readiness of these newborns for initiation of enteral feeding.
In addition to the limitations intrinsic to the technique of splanchnic oximetry monitoring, whose registered values exhibit a variability of up to 16%, there are other limitations to our study. We did not have a large enough sample to detect a significant association between oximetry data and oral feeding tolerance, and since the design was not double-blind, we could not prevent the risk of bias resulting from oximetry values influencing nutritional intervention in individual patients. To prevent bias resulting from confounding variables such as respiratory or haemodynamic support in immature newborns in the first days of life, we did not evaluate newborns delivered before 32 weeks’ gestation and therefore excluded many newborns that had experienced early, and therefore more severe, placental insufficiency, although in any case oral feeding tolerance could not be evaluated in many of these patients, as they often require prolonged parenteral nutrition. A more complex and comprehensive study is required to assess the correlation between placental insufficiency and abdominal oximetry in patients delivered before 32 weeks’ gestation.
We believe that our study offers a novel perspective in assessing the correlation between abdominal oximetry data and foetal Doppler ultrasound findings in SGA patients. Prenatal Doppler ultrasound examinations are becoming increasingly thorough in the assessment of the haemodynamic status of the foetus, yet few perinatal studies have assessed the correlation of this information with neonatal monitoring data.
In conclusion, our study found a correlation between foetal Doppler ultrasound findings and the splanchnic oximetry pattern in the first days post birth. We found that infants with a foetal history of mild placental insufficiency or stage I IUGR, with mild or no blood flow redistribution, did not differ significantly from infants with LBW in their oximetry values or oral tolerance. On the other hand, newborns that had experienced moderate or severe placental insufficiency had abnormal oximetry values. The magnitude of the decrease in oxygen saturation was associated with the severity of placental insufficiency, and these patients were at higher risk of feeding intolerance.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fuentes Carballal J, Avila-Alvarez A, Taboada Perianes M, Martínez Regueira S, Fernández Trisac JL. Oximetría esplácnica en neonatos pequeños para la edad gestacional en relación con el estudio doppler prenatal. An Pediatr (Barc). 2020;92:253–61.