Sleep apnoea-hypopnoea syndrome (SAHS) and childhood obesity are two high prevalence conditions that represent a public health challenge.
ObjectiveTo analyse the association between both and comparing child groups that had or did not have both conditions.
Patients and methodsA prospective study in children (3–14 years), referred to the “Multidisciplinary Sleep Unit” due to suspected SAHS, between 1 November 2015 and 1 August 2017. The following parameters were evaluated: anthropometry, symptoms, blood pressure, ear, nose, and throat examination, polysomnography (nocturnal PSG) and laboratory tests.
ResultsA total of 67 children were evaluated (64% non-obese (NOb) and 36% obese (Ob). It was observed that the Ob were older (P < .001), slept less hours (P = .028), did less physical exercise (P = .029), ate less in the school dining room (P = .009), had la lower sleep efficiency, and had abnormal values in carbohydrate and lipid metabolism.
The children with SAHS were younger (P = .010), a high percentage of daytime sleepiness (P = .001), and breathing through the mouth (P = .006), greater percentile of diastolic blood pressure (P = .019) and a lower IGF-1 (P = .003) than those that did not have SAHS. The comparison of the SAHS NOb and SAHS Ob groups, showed that the first group were younger (P = .010), snored more (P = .012), had a more severe SAHS (IAH 13.1 vs 5.4, P = .041), and a higher GOT (P < .001). In the second group, they slept less hours P = .038) and showed lower values of glucose (P = .039), insulin (P < .001), and HOMA (P < .001).
ConclusionThe behaviour of SAHS is different in obese children and non-obese children, with differences in age, clinical characteristics, severity of SAHS, and metabolic changes. The children diagnosed with SAHS were in the higher percentile of diastolic blood pressure. Obesity was associated with worse sleep quality, and changes in carbohydrate and lipid metabolism.
El síndrome de apneas-hipopneas del sueño (SAHS) y la obesidad infantil son dos entidades con alta prevalencia que constituyen un problema de salud pública.
ObjetivoAnalizar la interacción entre ambas, comparando grupos de niños que presentaban o no ambas condiciones.
Pacientes y metodologíaEstudio prospectivo en niños (3–14 años), remitidos a la "Unidad Multidisciplinar de Sueño" por sospecha de SAHS, entre el 1/11/2015 y el 1/08/2017. Se evaluaron los siguientes parámetros: antropometría, síntomas, tension arterial, exploración otorrinolaringológica, polisomnografía (PSG nocturna) y studio analítico.
ResultadosSe valoraron 67 niños, 64% no obesos (NOb) y 36% obesos (Ob).
Se observó que los Ob tenían más edad (P < ,001), dormían menos horas (P = ,028), realizaban menos ejercicio físico (P = ,029), comían menos en comedor escolar (P = ,009), tenían menor eficiencia del sueño y presentaban valores alterados en el metabolismo hidrocarbonado y lipídico.
Los niños que presentaba SAHS tenían menor edad (P = ,010), un mayor porcentaje de somnolencia diurna (P = ,001) y respiración bucal (P = ,006), mayor percentil de tensión arterial diastólica (P = ,019) y menor IGF-1 (P = ,003) que los que no presentaban SAHS.
La comparación de los grupos de SAHS NOb frente SAHS Ob, reveló que los primeros eran de menor edad (P = ,010), roncaban más (P = ,012), tenían mayor severidad del SAHS (IAH 13.1 vs 5.4, P = ,041) y mayor GOT (P ≤ ,001) y en el segundo grupo, se objetivó que dormían menos horas (P = ,038) y mostraban valores mayores de glucosa (P = ,039), insulina (P < ,001) y HOMA (P < ,001).
ConclusiónEl comportamiento del SAHS es diferente en los niños con y sin obesidad, presentando diferencias en la edad, características clínicas, severidad del SAHS y alteraciones metabólicas. Los niños con diagnóstico de SAHS tienen mayor percentil de tensión arterial diastólica. La obesidad conlleva una peor calidad de sueño y alteraciones en el metabolismo hidrocarbonado y lipídico.
Sleep-related breathing disorders are very frequent in the paediatric population and are associated with important repercussions on health, such as changes in growth, metabolic, cardiovascular and neurocognitive disorders and impaired quality of life.
Sleep apnoea-hypopnoea syndrome (SAHS) is the most prevalent sleep-related breathing disorder and affects 2%–4% of children aged 2–6 years, a period that coincides with the peak of physiological adenotonsillar hypertrophy,1 which is the most frequent cause of paediatric SAHS.
The diagnosis of SAHS is based on clinical suspicion, the history and the findings of the physical examination, and its confirmation requires performance of a sleep study, respiratory polygraphy or overnight polysomnography (PSG).2
On the other hand, there is an association between obesity and SAHS in adults that is starting to be seen in children with increasing frequency. The NANOS study in children with obesity in Spain found a high prevalence of SAHS that ranged from 21.5% to 39.5%3.
The global prevalence of childhood obesity has reached the proportion of an epidemic and has become a public health problem.4 As occurs in the rest of the world, prevalence of obesity in Spain continues to grow, the most recent ALADINO study in children aged 6–9 years identified obesity in 20.4% of boys and 15.8% of girls.5
Obesity and SAHS each cause significant morbidity independently. Our aim was to assess the potential interaction between the two, which would be evinced by the presence of clinical, polysomnographic and metabolic differences between obese and non-obese patients with SAHS.
Thus, we conducted a study with the objective of describing the characteristics of children referred to a sleep unit for suspected SAHS. The study was conducted in a cohort of children with and without obesity, allowing us to analyse the differences between several groups: obese (Ob) versus non-obese (NOb), with SAHS versus without SAHS and obese with SAHS (SAHS-Ob) versus obese without SAHS (SAHS-Nob).
Sample and methodsWe conducted a prospective study in children of both sexes aged 3–14 years referred for suspected SAHS to the Multidisciplinary Sleep Unit of a tertiary care university hospital after receiving the approval of the Research Ethics Committee. We obtained the informed consent of the parents and/or legal guardians of every participant as well as the assent of all children aged more than 12 years. We assigned a code to each participant to guarantee the anonymity and confidentiality of the data.
The sample was obtained by consecutive enrolment of children referred for clinical suspicion of SAHS (presence of snoring and/or pauses in breathing during sleep) that met the inclusion criteria and did not meet any exclusion criteria. The inclusion criteria were: 1) patients referred from a primary care paediatrician aged 3–14 years; 2) signed informed consent. We excluded children with craniofacial anomalies, dysmorphic syndromes, psychomotor delay or genetic/neuromuscular disorders as well as children with a history of adenotonsillectomy or previous treatment of SAHS and children for whom we did not obtain signed informed consent.
We defined obesity as a body mass index (BMI) at or above the 95th percentile for age and sex (Fundación Orbegozo 2004 growth tables).6
We divided the sample in 2 groups based on the presence or absence of obesity: obese group (Ob) and non-obese group (NOb).
The following protocol was implemented in all children: 1) documentation of demographic and clinical characteristics (sex, ethnicity, perinatal history). 2) General medical history and sleep questionnaire: Pediatric Sleep Questionnaire, validated abridged version in Spanish (Chervin questionnaire).7 A score of 0.33 or higher was considered suggestive of SAHS.8 3) Physical examination with documentation of anthropometric measurements: weight (kg), height (m), BMI (kg/m2) and the corresponding percentiles6; morning blood pressure (BP) measured with an automatic monitor. Blood pressure was measured 3 times, and the mean of the last 2 measurements was used for the analysis of BP percentiles.9 4) Ear, nose, throat (ENT) examination with assessment of adenoid and tonsillar hypertrophy. Tonsil size was graded on a scale from 0 to IV based on the size and the percentage of the of the lateral dimension of the oropharynx occupied by the tonsils. Grade 0: absence of tonsils; Grade I: tonsils occupy 0%–25% of lateral dimension of oropharynx; Grade II: 25%–50%; Grade III: 50%–75%; Grade IV: 75%–100%.10 The adenoids were examined by anterior rhinoscopy and with a fibre optic endoscope.
All children underwent a nocturnal laboratory-based PSG in the Multidisciplinary Sleep Unit, which included the use of electroencephalography, electrooculography (both eyes), electromyography (tibialis and submentalis), electrocardiography, monitoring of oronasal air flow through a thermistor channel, plethysmography for monitoring of thoracic and abdominal movements, position sensors to monitor body position, monitoring of oxygen saturation and heart rate by pulse oximetry, monitoring of snoring and air flow through pressure transducer channels in nasal prongs and continuous transcutaneous carbon dioxide (CO2) monitoring. We applied the criteria published in 2012 by the American Academy of Sleep Medicine (AASM) for scoring of sleep stages and associated events.11 We expressed the relative duration of each sleep stage as the percentage (%) of the total duration of sleep. We defined apnoea index (AI) and the apnoea-hypopnoea index (AHI) as the number of apnoeas and the number of apnoeas and hypopnoeas per hour of sleep, respectively. We also calculated the obstructive AHI (oAHI), central AHI (cAHI) and respiratory disturbance index (number of apnoeas, hypopnoeas and respiratory effort-related arousals [RERAs] per hour of sleep).
In adherence with the criteria established in the national consensus document for SAHS in children,2 the diagnosis of SAHS and indication of treatment were based on the AHI being 3 or greater, whether or not obstructive hypopnoea had been detected by PSG.
We performed laboratory tests for inflammatory, metabolic and endocrine markers. Blood samples were collected the morning after the PSG.
Statistical analysisWe used the software SPSS version 22 (Chicago, IL, USA). We have expressed quantitative data as median and interquartile range and categorical data as absolute frequency and percentage distributions in comparison with another dichotomous variable. We compared continuous data using the Mann-Whitney U test and categorical data with the chi square test or Fisher exact test. We defined statistical significance as a P-value of .05 or less.
ResultsBetween 1/11/2015 and 1/08/2017, 67 children enrolled in the study, 60% boys and 40% girls, referred for clinical suspicion of SAHS to the Multidisciplinary Sleep Unit. The median age was 5.5 years (interquartile range, 4.3–7.7). Most participants were Caucasian (86%), while 13% were Latin American and 2% Asian.
Sixty-four percent of participants (43) were obese, and 36% (24) were not obese. Tonsillar hypertrophy was detected in 93% of children (grades II-III-IV), snoring in 91% and mouth breathing in 60%.
In this case series, 14 patients had a family history of SAHS (16% in the father, 2% in the mother and 5% in siblings). The findings of the family history of metabolic disorders were: obesity in the father in 28%, the mother in 17% and a sibling in 4% of participants; dyslipidaemia in the father in 16%, the mother in 5% and a sibling in 2% of participants; high BP in the father in 6% and the mother in 5% of participants.
The results of the Chervin questionnaire for detection of sleep disorders were positive in 70% of the patients. Based on the findings of the PSG, 39% of participants had SAHS without obstructive hypopnoea, 4% SAHS with obstructive hypopnoea and 24% reduced air flow without meeting criteria for SAHS, while 33% did not have respiratory problems.
When it came to management, 39% of patients underwent an adenotonsillectomy and 1% an adenoidectomy, 18% were treated with sleep hygiene and dietary measures, 5% received pharmacological treatment (nasal steroids and antileukotrienes) and 25 patients remained in follow-up for monitoring of their condition.
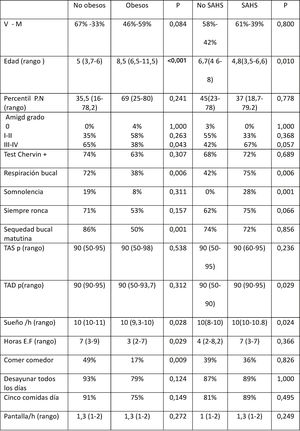
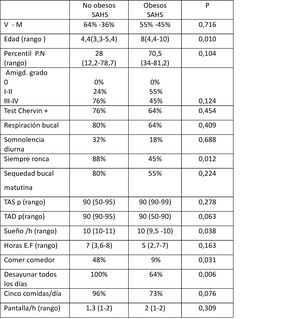
Comparison of children with and without obesityThe comparison of children with obesity (Ob, n = 24) and children without obesity (NOb, n = 43) (Table 1), revealed a significantly younger age, higher prevalence of mouth breathing, higher prevalence of morning dry mouth and greater tonsil size in the NOb group, with no significant differences based on sex, birth weight or score in the Chervin questionnaire.
General characteristics of children without versus with obesity and without versus with SAHS.
AHI, apnoea-hypopnoea index; BW, birth weight; DBP, diastolic blood pressure; F, female; h, hours; M, male; PA, physical activity; pct, percentile; SAHS: sleep apnoea-hypopnoea syndrome; SBP, systolic blood pressure; SAHS, sleep apnoea-hypopnoea syndrome.
In the Ob group, 50% of children had obese fathers and 30% obese mothers, compared to 17% of fathers and 10% of mothers in the NOb group (P = .005 y P = .032).
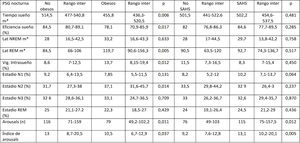
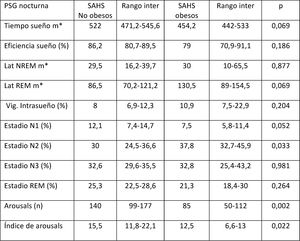
There were also significant differences in the comparison of sleep hygiene and dietary habits in the Ob and NOb groups (Table 1), as Ob slept fewer hours, exercised fewer hours a week and were less likely to use the school lunch services. The comparison of the nocturnal PSG parameters in the Ob vs NOb groups (Tables 2 and 3) revealed that obese children had a shorter total sleep duration TTS, a lower sleep efficiency, fewer arousals, lower REM AHI and cAHI, a longer REM latency and higher proportions of intervening wakefulness and stage 2 sleep.
Respiratory polysomnographic parameters.
AHI, apnoea-hypopnoea index; Ap, apnoea; cAHI, central apnoea-hypopnoea index; NREM AHI, non-REM sleep apnoea-hypopnoea index; oAHI, obstructive apnoea-hypopnoea index; ODI, oxygen desaturation index; PETCO2, partial pressure of end tidal CO2; RDI, respiratory disturbance index; REM AHI, REM sleep apnoea-hypopnoea index; RERA, respiratory event-related arousal; SAHS, sleep apnoea-hypopnoea syndrome; SatO2, oxygen saturation.
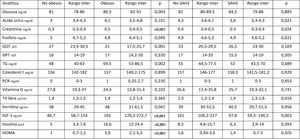
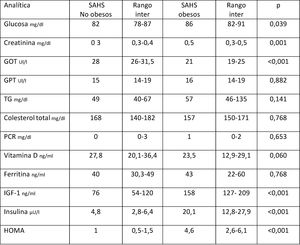
When it came to metabolic parameters (Table 4), we found higher levels of glucose, creatinine, triglycerides (TG), basal insulin, ferritin and insulin-like growth factor-1 (IGF-1) levels and a higher homeostatic model assessment (HOMA) index (indicator of insulin resistance) in children with obesity.
Metabolic parameters in children without vs with obesity and without vs with SAHS.
ALT, alanine aminotransferase; AST, aspartate transaminase; CPR, C-reactive protein; HOMA, insulin resistance index; IGF-1, insulin-like growth factor-1; IQR, interquartile range; SAHS, sleep apnoea-hypopnoea syndrome; TG, triglycerides.
When we compared children with SAHS (n = 36) and without SAHS (n = 31) (Table 1), we found that the SAHS group was significantly younger, had a significantly higher prevalence of daytime somnolence and of mouth breathing and had diastolic blood pressures (DBP) at significantly higher percentiles compared to the group without SAHS. We did not find significant differences between these groups in sex distribution, tonsil size or the results of the Chervin questionnaire.
The comparison of sleep hygiene and dietary habits (Table 1), polysomnographic parameters (Tables 2 and 3) and metabolic parameters (Table 4) revealed significant differences between children with and without SAHS groups. Children with SAHS slept more hours at night, had more arousals and a higher arousal index (without significant differences in any other neurophysiological parameter), exhibited respiratory PSG parameters characteristic of SAHS and had lower levels of uric acid, creatine and IGF-1 and higher levels of free thyroxine (T4).
Analysis of SAHS in obese versus non-obese childrenOf all children with a diagnosis of SAHS (n = 36), 25 (69.4%) were not obese and 11 (30.6%) were obese.
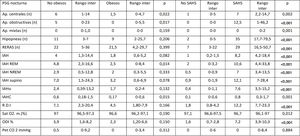
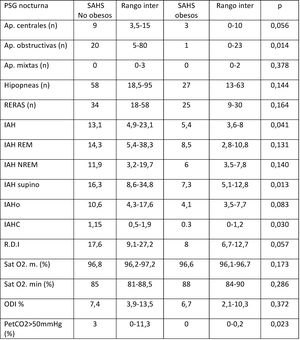
Table 5 presents the characteristics of the SAHS-NOb and the SAHS-Ob groups; children in the SAHS-NOb group were younger and more likely to snore, differences that were significant in comparison with the SAHS-Ob group.
As for sleep hygiene and dietary habits (Table 5), children in the SAHS-Ob group slept fewer hours, and were less likely to use school lunch services and to have breakfast every day. When it came to PSG parameters (Tables 6 and 7), children in the SAHS-NOb group experienced more arousals and spent less time in stage 2 sleep relative to the total sleep duration, and had significantly higher AHI, supine AHI, central AHI and AI values and a significantly higher frequency of PETCO2 greater than 50 mmHg compared to the SAHS-Ob group, and when it came to metabolic parameters (Table 8), children in the SAHS-Ob group had significantly higher glucose, creatinine, IGF-1, insulin and HOMA index values, and lower aspartate transaminase (AST) levels compared to the SAHS-NOb group.
Respiratory polysomnographic parameters in the SAHS-non obese versus SAHS-obese subgroups.
AHI, apnoea-hypopnoea index; Ap, apnoea; cAHI, central apnoea-hypopnoea index; NREM AHI, non-REM sleep apnoea-hypopnoea index; oAHI, obstructive apnoea-hypopnoea index; ODI, oxygen desaturation index; PETCO2, partial pressure of end tidal CO2; RDI, respiratory disturbance index; REM AHI, REM sleep apnoea-hypopnoea index; RERA, respiratory event-related arousal; SAHS, sleep apnoea-hypopnoea syndrome; SatO2 min, minimum oxygen saturation.
Metabolic parameters in the SAHS-non obese versus SAHS-obese subgroups.
ALT, alanine aminotransferase; AST, aspartate transaminase; CPR, C-reactive protein; HOMA, insulin resistance index; IGF-1, insulin-like growth factor-1; IQR, interquartile range; SAHS, sleep apnoea-hypopnoea syndrome; TG, triglycerides.
We conducted a prospective study to assess the clinical, polysomnographic and metabolic characteristics of a clinical cohort of children with and without obesity referred for suspected SAHS. In this cohort, children with SAHS had a higher morning DBP, and the Chervin test could not discriminate between children with and without SAHS. In addition, children with obesity had abnormal sleep patterns and poor sleep hygiene and dietary habits, were more likely to have a family history of obesity and had higher glucose, insulin, HOMA index and triglyceride values.
Short sleep duration is associated with an increased risk of obesity,12,13 and the prevalence of sleep disorders has been found to be higher in obese children.14 In the sample under study, obese children exhibited sleep disturbances (shorter duration, lower efficiency, longer REM latency). At the same time, sleep deprivation causes daytime somnolence and fatigue that may result in a decrease in physical activity, which is consistent with the hours of physical activity reported by children in each group. Recent studies have analysed the association between sleep duration and lifestyle habits. Konstantions et al.15 found that insufficient sleep in children was associated with unhealthy dietary habits. The use of school lunch services has emerged as a protective factor against obesity. In our study, the proportion of children that used school lunch services was lower in the Ob group.5,16,17
Several studies have found a clear association between the BMI of parents and the BMI of children,18,19 which was corroborated by our findings, as obesity in parents was more frequent in the obese group, which highlights the importance of this factor.
There are metabolic abnormalities associated with childhood obesity from an early age that present in isolation or as part of what is known as metabolic syndrome, as demonstrated by a collaborative study published by Martos et al.20 Our study also found differences in parameters of carbohydrate metabolism (glucose and insulin levels and HOMA index) and lipid metabolism (TG levels), with higher values in obese children.
The use of questionnaires for screening of SAHS has been studied extensively. We used a validated version of the Chervin questionnaire in Spanish. In the sample under study, the questionnaire results were positive in 72% of children with SAHS compared to 68% of children without SAHS (P = .689), which brings into question the use of questionnaires for diagnosis; in this regard, a recent meta-analysis analysed the accuracy of the Chervin test21 and found that it offered a lower sensitivity and specificity than previously reported and that it was not useful for assessment of individuals with obesity, in agreement with other studies.3
Of all the health problems associated with SAHS, cardiovascular disease is among the most important, and the association between SAHS and high BP in children has been subject to extensive study, although the data published to date are contradictory. In our study we found significantly higher morning DBP values in children with SAHS. Similarly, several authors have found that BP values increase with the severity of SAHS.22,23 Furthermore, studies that used 24-h ambulatory BP monitoring found an absence of the nocturnal dipping in BP and a marked increase in the morning BP associated with SAHS,24,25 and therefore a higher cardiovascular risk.
On the other hand, the comparison of neurophysiological parameters in children with and without SAHS only found differences in the number of arousals, which is why use of respiratory polygraphy, which does not monitor these parameters, is currently recommended for diagnosis of SAHS.26
Another important aspect is the association between SAHS and abnormal growth; in this regard, a study conducted in children with SAHS and with primary snoring found lower concentrations of insulin-like growth factor binding protein-3 (IGFBP-3), a transport protein, although it found no differences in the concentration of IGF-1.27 When we compared children with and without SAHS in our study, we only found differences in the levels of IGF-1, creatinine, uric acid and free T4, although these differences could be explained by the age difference between the groups.
In the analysis of the SAHS group, we found that the SAHS-NOb subgroup was younger in age, had a higher prevalence of regular snoring and had a higher AHI compared to the SAHS-Ob subgroup. We did not find differences in tonsil size between groups, although a grade III-IV size was observed in 76% of the SAHS-NOb subgroup compared to 45% of the SAHS-Ob subgroup, which may be explained by the higher degree of adenotonsillar hypertrophy in the younger age group documented in the literature.1,2 Still, the fact remains that not all children with adenotonsillar hypertrophy develop SAHS.
Although SAHS has been identified as a risk factor for insulin resistance in adults, the association in children unclear. Studies performed mainly in adolescents have found an association between metabolic syndrome and SAHS, whereas in younger children, SAHS is associated with an increase in insulin resistance when SAHS co-occurs with obesity.2,28 In our study, we found more carbohydrate metabolism abnormalities in the SAHS-Ob group, abnormalities that were present in the Ob group, suggesting that insulin resistance is mainly determined by obesity with a lesser contribution of SAHS, as described by other authors.29 We also found a higher median HOMA index in the SAHS-Ob group compared to the subset of children with obesity and without SAHS, which suggests that SAHS enhances the effect of obesity, a phenomenon observed in previous studies with larger samples.30
Another salient finding was the difference in liver enzyme levels in the comparison of the SAHS-NOb and SAHS-Ob subgroups, with higher levels in the non-obese subgroup (P < .001), which also had a higher AHI (P = .041). Our findings on this aspect are consistent with the previous literature, as the presence and severity of SAHS is associated with liver enzyme elevation and non-alcoholic fatty liver disease independently of the BMI.31
One potential limitation of the study is that it was conducted in a cohort of children with and without obesity referred for suspected SAHS, so that they were not representative of the entire paediatric population with obesity, in which SAHS is subject to a low level of suspicion and underdiagnosed, as evinced by the previous literature.3 Another limitation is the small sample size in the analysis of the SAHS-NOb vs SAHS-Ob subgroups. In relation to this, another possible limitation is the reason for referral: the presence of snoring. In our study, snoring had been documented at the time of referral in 62% of children without SAHS vs 75% of children with SAHS (P = .066), while in the sleep study (snoring detected by pressure transducers and/or witnessed in the laboratory) it was detected in 45% of children without SAHS vs 77% of children with SAHS (P = .017), so that while snoring is a warning sign, emphasis must be placed on identifying other symptoms suggestive of SAHS, especially in older or obese children.
ConclusionsOur study found that SAHS presented differently in 2 paediatric phenotypes, with and without obesity, with different findings based on age and clinical, polysomnographic and metabolic characteristics.
Children with obesity had abnormal sleep patterns and poor sleep hygiene and dietary habits, in addition to abnormalities in carbohydrate and lipid metabolism.
We found an increased DBP in children with SAHS, which is indicative of an increased cardiovascular risk.
Our findings also highlight the need to improve the questionnaires used to detect SAHS and to develop specific instruments to assess paediatric patients with obesity.
Thus, it is important to be aware of the increased risk of morbidity associated with SAHS in the presence of obesity, and training programmes and awareness campaigns should be implemented at the primary care level to improve knowledge of SAHS in the context of childhood obesity. Such programmes would counteract the tendency to underdiagnose SAHS, contributing to its early treatment and therefore to reducing the morbidity associated with this condition.
FundingBiomedical research grant from the Regional Department of Health of Castilla y Leon, file no. GRS 1116/A/15, project title “El síndrome de apneas-hipopneas durante el sueño en población infantil obesa y no obesa, implicaciones metabólicas”.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank children and their parents for their participation in the study. We are also grateful for the legacy, constant encouragement and contribution to this work of the late Dr. Joaquín Terán Santos.
Please cite this article as: Martínez Cuevas E, Muñoz Peláez C, Ordax Carbajo E, Navazo Eguia AI, Martín Viñe L, Prieto Jimeno A, et al. Síndrome de apneas-hipopneas durante el sueño en obesos y no obesos: características clínicas, polisomnográficas y metabólicas. An Pediatr (Barc). 202;95:147–158.
Previous presentations. This study has been presented in the following congresses and meetings: 67th Congress of the Asociación Española de Pediatría, June 6–8, 2019, Burgos, Spain; XXVI Annual Meeting of the Sociedad Española de Sueño, April 26–28, 2018, Barcelona, Spain; 52nd Congress of the Sociedad Española de Neumología y Cirugía Torácica, June 13–16, 2019, Santiago de Compostela, Spain.