This is a Consensus document of the Spanish Society of Paediatric Infectious Diseases (Sociedad Española de Infectología Pediatrica), the Spanish Society of Paediatric Rheumatology (Sociedad Española de Reumatología Pediátrica) and the Spanish Society of Paediatric Orthopaedics (Sociedad Española de Ortopedia Pediátrica), on the treatment of uncomplicated acute osteomyelitis and septic arthritis.
A review is presented on the medical and surgical treatment of acute osteoarticular infection, defined as a process with <14 days of symptomatology, uncomplicated and community-acquired. The different possible options are evaluated based on the best available scientific knowledge, and a number of evidence-based recommendations for clinical practice are provided.
Presentamos el documento de Consenso sobre tratamiento de la osteomielitis aguda y la artritis séptica no complicadas, elaborado por la Sociedad Española de Infectología Pediátrica, la Sociedad Española de Reumatología Pediátrica y la Sociedad Española de Ortopedia Pediátrica.
En este documento se revisa el abordaje y el tratamiento médico-quirúrgico de la infección osteoarticular aguda, considerada como aquella que presenta una evolución inferior a 14 días, no complicada, de origen comunitario en niños, basándonos en las mejores evidencias científicas disponibles y valorando las diversas opciones disponibles en la actualidad. En función de dichas evidencias, se aportan una serie de recomendaciones para la práctica clínica.
This is the second part of an earlier document, which examined the aetiopathogenesis and diagnosis of osteoarticular infections (OAI). This part addresses treatment, where there have been numerous new contributions in recent years,1–10 as well as follow-up and prognosis. It includes both acute osteomyelitis (AOM) and septic arthritis (SA), and basically reviews haematogenous community-acquired infections with acute course (<14 days of symptoms).
At the end of the document the main recommendations for each section are listed, together with the level of evidence and degree of recommendation, as defined in Table 1.11
Level of evidence and strength of recommendation used in this consensus document.
| Category | Definition |
|---|---|
| Strength of recommendation | |
| A | Good evidence |
| B | Moderate evidence |
| C | Poor evidence |
| Quality of evidence | |
| I | Properly randomised clinical studies |
| II | Well designed but non-randomised clinical studiesCohort studiesCase and control studiesOthers: multiple series or consequence of convincing results of non-controlled experiments |
| III | Opinion of experts based on clinical experienceDescriptive studiesRecommendations of committees of experts |
Modified from Khan et al.11
The aetiology was described in the previous document, although it is briefly set out in Table 2.
Most common aetiology of osteoarticular infection by age and associated risk factors.
| Age | Bacteria |
|---|---|
| <3 monthsa | S. aureusS. agalactiaeEnterobacteria (especially Escherichia coli) |
| 3 months–5 yearsb | S. aureusK. kingaeS. pyogenes |
| >5 yearsc | S. aureusS. pyogenes |
| Risk situation | Bacteria |
|---|---|
| Puncture wound in foot wearing training shoes | P. aeruginosa |
| Chicken pox and wounds | S. pyogenes |
| Sickle-cell disease | Salmonella sp. |
| Complement deficiency | Neisseria meningitidisd |
| Newborn with complex pathologies, immunodeficiencies, patients with prostheses or osteosynthesis material | Plasmocoagulase-negative Staphylococcus; S. epidermidis, S. hominis, S. saprophyticus, S. haemolyticus, S. lugdunensis. Candida sp., as well as other gram-positive cocci and bacilli and gram-negative bacilli |
| Agammaglobulinaemia | Mycoplasma pneumoniae |
| Chronic granulomatous disease | S. aureus, Serratia marcescens and Aspergillus fumigatus, among others |
| Patients from countries highly endemic for tuberculosis, immunodeficiencies that affect the interferon-gamma/interleukin-12 axis and treatments with biologic immunomodulators that interfere with interferon production | Mycobacterium tuberculosis |
Other microorganisms occasionally associated with osteoarticular infection in newborns are Neisseria gonorrhoeae, coagulase-negative Staphylococcus and Candida.
Within Staphylococcus aureus a distinction can be drawn between strains sensitive to methicillin (MSSA) and those resistant to methicillin (MRSA) through modification of the penicillin-binding proteins, and among these, between community-acquired (CA-MRSA) and hospital-acquired strains. In Spain most infections in children are caused by MSSA (>90%); however, we need to take account of the high rate of CA-MRSA in other geographical areas, such as certain states in the United States, Latin America, North Africa and Eastern Europe,12 which will have to be considered in children from these regions. Methicillin resistance is an indicator of resistance to the other β-lactam antibiotics, including cephalosporins (except ceftaroline) and carbapenems.
CA-MRSA tends to have few associated antibiotic resistances and is normally sensitive to clindamycin, cotrimoxazole (TMP–SMX), glycopeptides (vancomycin and teicoplanin), rifampicin and linezolid.12Kingella kingae is usually sensitive to β-lactam antibiotics, including ampicillin and cephalosporins, and therefore does not tend to give rise to treatment problems, except when clindamycin or cloxacillin are used in monotherapy. Other bacteria, such as Streptococcus pyogenes or Streptococcus pneumoniae normally respond well to penicillin.
TreatmentChildren with OAI should be hospitalised for initial assessment and intravenous (IV) antibiotic treatment. These infections call for a multidisciplinary approach involving orthopaedic surgeons, rheumatologists and paediatric infectious diseases specialists, according to each case.
Surgical treatmentSeptic arthritisThe classic treatment for all kinds of SA involves performing an arthrotomy (surgical drainage) to evacuate the joint, washing out the purulent material, placing an external drain to avoid reaccumulation of fluid and immobilising the joint to avoid subluxations, especially in the hip.13 Draining of the affected joint and lavage is universally accepted, but it is not so obvious which is the best way of achieving it (arthrotomy, arthroscopy or arthrocentesis), since there are no adequate studies endorsing any particular approach, though traditionally arthrotomy has been recommended in the shoulder, and especially the hip, given the greater risk of sequelae.13 The need for surgical drainage is always more likely to arise in infections by high-virulence microorganisms, such as S. aureus producing toxins like Panton–Valentine leukocidin (PVL) (generally MSSA, sometimes MRSA), and when the evolution of the infection is more prolonged.13
Arthrocentesis: joint puncture, needle aspiration and joint lavagePerforming an arthrocentesis on the affected joint is essential in order to obtain a microbiological diagnosis, achieve decompression of the joint space (avoiding vascular compromise in the shoulder and the hip) and facilitate the effectiveness of the antibiotic after the purulent material has been flushed out.14 It must be undertaken in aseptic conditions, and is a simple procedure (more complex in the hip and the shoulder), with few risks, which can guide diagnosis.15 There are no absolute contraindications, except local infection at the puncture site, severe sepsis or shock. It must be performed as soon as possible (preferably before starting the antibiotic), although it can be delayed for a few hours (6–12h, for example); early intervention is especially important in SA of the hip and shoulder.14,16 Most of the authors of this document are in favour of draining the joint and starting antibiotic therapy as soon as possible, but cannot give an exact recommendation of the time needed to avoid complications or sequelae.
Ultrasound can be very helpful for locating the puncture site. The child must receive appropriate sedoanalgesia; inhaled nitrous oxide may be administered.
Both arthrocentesis and arthrotomy enable the joint to be washed out with normal saline. Arthrocentesis has the advantage of being a less traumatic procedure and achieving more rapid patient recovery, and is associated with a faster decrease in C-reactive protein (CRP), which could reduce the duration of IV antibiotic and length of stay in hospital.
Prompt performance of arthrocentesis, daily clinical assessment and repetition of the procedure when necessary with joint lavage are the keys to the success of this therapeutic approach.17
Therapeutic arthrocentesis can be used for any joint, including the shoulder and the hip, as various studies show.1,4,5,17–20 In a randomised study of children with arthritis of the shoulder, for example, no differences were found in prognosis or length of stay in patients treated with aspiration versus arthrotomy.1 As for the hip, there are also studies that support a favourable outcome in children treated with aspiration/irrigation.5 The following are factors that the authors consider indicate a poor prognosis and thus a need for open surgery: symptoms lasting at least six days, CRP >10mg/dL, >15,000neutrophils/mm3 and erythrocyte sedimentation rate (ESR) >50mm/h.5 Repeated aspiration of the hip joint has also been assessed, and more rapid recovery and resumption of walking were observed, without sequelae,19 though some patients required open drainage.
In conclusion, in most cases, except in newborns (NBs), where there is no adequate evidence of outcomes without surgical arthrotomy,18 children with recent symptoms (<5–6 days) could be candidates for drainage via arthrocentesis and antibiotherapy.4,6,18 In SA of the shoulder and hip, the decision will depend on how early action is taken, on the analytical assessment and on the experience of the team responsible for the patient. In all these cases, the children should be admitted to a hospital with an experienced orthopaedic surgeon, to perform surgical treatment if necessary.
ArthrotomyThis is the main advanced surgical procedure in the treatment of SA. In principle, it can be performed on any joint. There are authors who consider it essential for treating SA of the hip,13 although some more recent studies note the possibility of nonsurgical approaches in these locations.4,19 Equally, other authors recommend surgical intervention if the joint fluid is not satisfactorily drained after 2 or 3 arthrocenteses.17 Arthrotomy could be indicated, at the outset, in cases of longer evolution, given the greater difficulty of evacuating denser and more organised material,13,16 in cases of raised inflammatory markers or of highly virulent pathogens (MRSA) and in neonates and small infants.5,19 The object of the surgical procedure can be regarded as threefold21: drainage of the purulent content and necrotic material, reduction of the intra-articular pressure and direct assessment of the lesion, and also a collection of microbiological and anatomopathological samples. In addition, it enables an external drain to be placed to avoid further collections.13,22 Although no well-designed studies exist, many authors suggest leaving this type of drainage for irrigation/aspiration, especially in the hip; it must be removed promptly (<48–72h).
Given that this procedure involves opening the joint, it may be necessary, in some cases, to stabilise the joint using cutaneous traction or ferrules to avoid dislocations in the post-operative period, although early mobilisation must be implemented to avoid problems later, such as rigidity or flexion.13
Other authors propose arthroscopy as a less aggressive method than arthrotomy for treating SA in children.6,23,24 The main limitations are the age of the patients and the difficulty of the procedure.
Surgical drainage in osteomyelitisIt has been found that over 90% of patients with AOM have favourable outcomes with antibiotic treatment if initiated early,7,18,25,26 performing surgical drainage when the presence of a collection or sequestrum in the bone or subperiosteal level is detected,18 when no clinical improvement has occurred after 48–72h of antibiotherapy and in acute exogenous osteomyelitis (AEO). However, subperiosteal abscesses, even those greater than 3mm, may have favourable outcomes without surgical drainage.18 As in the case of SA, microbiological samples must be taken, as well as anatomopathological samples when this is considered necessary, and it is essential to place an external drain to avoid post-surgical collections.
It is worth emphasising that some experts have had very good results performing an initial bone puncture, which could improve aetiologic diagnosis and evolution.
Medical treatmentIn the last few years a trend has been emerging towards simplifying antibiotic treatment in uncomplicated OAI, with the use of parenteral antibiotic treatment followed by a course of oral antibiotics, with high doses of antibiotherapy and shorter duration, both of IV treatment8–10 and overall.2,3 This trend is based on the pioneering experience of a controlled clinical trial published by Peltola et al.,7 which has subsequently been confirmed in prospective cohort or randomised studies.2,3,7,8,10 Recently, guidelines from the United Kingdom incorporating some of these recommendations have been published.14 These strategies may not be valid in cases of highly virulent microorganisms, such as PVL-producing S. aureus, given their greater severity and poorer prognosis,27,28 and longer duration of antibiotic treatment is recommended.28
Initial empirical treatmentIf there is any suspicion of OAI in a child, IV antibiotic treatment should be initiated promptly (Table 3), after collecting samples for microbiological testing, as appropriate.25
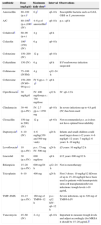
Antibiotics most commonly used in OAI in children (listed in alphabetical order).
| Antibiotic | Dose (mg/kg/d) | Maximum daily dosea | Interval | Observations |
|---|---|---|---|---|
| Amoxicillin | 80–100 (p.o.)i | 6gi | q6–8h | Susceptible bacteria such as GAS, GBS or S. pneumoniae |
| A/C | 80–100b (p.o.)100c (IV) | 4–6g of amoxicillind | q6–8h | p.o.: q8h |
| Cefadroxile | 60–90 (p.o.) | 4g | q8h | |
| Cefazolin | 100i (150) (IV) | 6g | q6–8h | |
| Cefotaxime | 150–200 (IV) | 12g | q6–8h | |
| Ceftazidime | 150 (IV) | 6g | q8h | If Pseudomonas infection suspected |
| Ceftriaxone | 75–100 (IV/IM) | 4g | q12–24h | |
| Cefuroxime | 150–200 (IV)60–90 (p.o.) | IV 6gp.o.: 3gi | q8h | |
| Ciprofloxacinf | 30mg/kg/d | IV: 400mg/dosep.o.: 750mg/dose | q12h | IV: q8–12h |
| Clindamycin | 30–40 (p.o./IV) | IV: 2.7gp.o.: 1350mg | q6–8h | In severe infections up to 4.8gr/d (IV) has been used |
| Cloxacillin | 150 (up to 200) (IV) | 12g | q4–6h | Not recommended p.o. as it does not have optimal bioavailability |
| Daptomycing | 4–10 (IV) | 4–6mg/kg(350 and 500mg vials) | q24h | Infants and small children could need larger doses:>12 years: 4–6mg/kg6–12 years: 7mg/kg2–6 years: 8–10mg/kg |
| Levofloxacinf | 10 (p.o./IV) | p.o.: 75mg IV: 500mg | q24h | ≤5 years: 10mg/kg/12h |
| Linezolid | 30 (p.o./IV) | 600mg/dose | q8h | ≥12 years: 600mg/12h |
| Rifampicin | 15–20 (p.o./IV) | 600mg/24h | q12–24h | Not in monotherapy |
| Teicoplanin | 6–10 | 400mg | q24h | First 3 doses: 10mg/kg/12hDoses of up to 15–20mg/kg/d have been used in patients with hematopoietic stem cell transplantationSevere infections: trough levels >10μg/mL |
| TMP–SMX | 10–15 (p.o./IV) | 160mg of TMP/6–12h | p.o.: q12hIV: q6–12h | Severe infections, up to 320mg of TMP/6h IV |
| Vancomycin | 45–60 (IV) | 2–4g | q6–8h | Important to measure trough levels and adjust accordingly (for MRSA it should be 15–20μg/mL)h |
A/C, amoxicillin/clavulanic acid; d, day; GAS, S. pyogenes; GBS, S. agalactiae; h, hours; IM, intramuscular; IV, intravenous; MRSA, methicillin-resistant S. aureus; p.o., by mouth.
Increasing the dose of amoxicillin IV to 120–150mg/kg/day could be considered, using formulations with a lesser quantity of clavulanic acid (amoxicillin:clavulanic concentration 10:1; 2g/200mg or 500mg/50mg vials).
Avoid administering >125mg of clavulanic acid per dose (by adding amoxicillin alone, if necessary). Consider administering a probiotic, especially if gastrointestinal side effects occur.
At the time of writing these guidelines the marketing of cefadroxil 250mg/5mL suspension had been suspended in Spain.
According to the data sheet, in patients <18 years ciprofloxacin for this indication and levofloxacin for any indication would be off-label, although there is ample experience in children.
Daptomycin is not approved for patients <18 years (compassionate treatment) and is not recommendable if pulmonary involvement due to septic emboli is suspected, as it is rendered inactive by the pulmonary surfactant. The youngest children could require 6mg/kg/12h.
An antibiotic with good activity against MSSA and S. pyogenes should be used, since these are the most common aetiological agents.2,10,27,30 In the case of AOM through puncture injury to the foot bone (through a training shoe), Pseudomonas aeruginosa should also be covered. In children aged >5 years of age it is advisable to use an antibiotic with good activity against K. kingae,29 and in children with <3 doses of the H. influenzae type b or S. pneumoniae vaccine (especially in those <2 years old) these microorganisms should also be covered.30 In regions where the prevalence of MRSA infections is <10% of S. aureus infections, an antibiotic with good coverage for this bacterium should be used.
The antibiotics, most widely used and with which there is most experienced in children are cefazolin, cloxacillin and clindamycin.25,30 This group of experts considers cefazolin the antibiotic of choice in properly vaccinated children aged >2 years in geographical areas where the prevalence of CA-MRSA infections is <10%. In children 2 years of age or older that have received <3 vaccine doses, treatment with cefuroxime is recommended, and as alternatives cloxacillin (with little activity against K. kingae)31 combined with cefotaxime or amoxicillin/clavulanic acid. In children younger than 3 months the recommendation is to combine cloxacillin and cefotaxime; cefazolin and gentamicin is also a suitable combination. Cloxacillin combined with ceftazidime would be the most appropriate antibiotic regime in AOM of the bones of the foot due to a puncture wound. These recommendations are set out in Table 4.
Initial empirical treatment of osteoarticular infections by age and certain underlying conditions of the patient.
| Age | Empirical antibioticsa |
|---|---|
| <3 months(including newborns) | Cloxacillin+cefotaxime/gentamicinaAlternative: consult paediatric infectious diseases specialist |
| 3 months–5 yearsb | Cefuroxime in monotherapy or cloxacillin+cefotaximecAlternative: A/CAlternatives in children >2 years with no suspicion of S. pneumoniae:Cefazolin or cloxacillind |
| 5 years | Cefazolin or cloxacillin |
| Adolescentse | Penicillin G (25,000U/kg/6h) IV or ceftriaxone IV/IM |
| Special situations |
|---|
| Sickle-cell anaemia: cloxacillin+cefotaxime or A/C in monotherapy |
| Anaerobes: clindamycin (alternatives: A/C or metronidazole)f |
| History of puncture wound: cloxacillin+ceftazidime |
| Prosthesis-related superinfection: vancomycin/linezolid/ciprofloxacin/levofloxacin with or without rifampicin |
| β-Lactam allergy: clindamycin, and as alternatives, TMP–SMX or quinolones. Combining rifampicin with either of them could be considered. For other options it would be advisable to consult a paediatric infectious diseases specialist. In such cases one should remember the possibility of K. kingae in children aged <2–5 years and enterobacteria in those <3 months, who might not be adequately covered with these antibiotics |
| Serious conditionsg |
| Glycopeptide (or linezolid)±rifampicin±clindamycin |
| Alternative: daptomycin (not approved in children) when glycopeptides or linezolid cannot be used, if there is no pulmonary involvement. |
A/C, amoxicillin/clavulanic acid; IM, intramuscular; IV, intravenous; MRSA, methicillin-resistant S. aureus; TMP–SMX, trimethoprim–sulfamethoxazole.
In children >2 years the same regimen could be used as in those >5 years, provided they are properly vaccinated, given that both Kingella and S. pneumoniae are uncommon.
If Kingella is suspected, combining another β-lactam antibiotic should be considered (as when clindamycin is used in monotherapy).
The empirical treatment would be the same as for children >5 years and this option would only be for suspected N. gonorrhoeae.
Always consider anaerobes in the event of torpid progression. For example, Fusobacterium necrophorum has sometimes been implicated.
Children with involvement of several locations, with associated sepsis or with pulmonary thromboembolisms. As long as there is no diagnostic confirmation of an MRSA, the possibility of adding a β-lactam antibiotic should be assessed, given that they have greater activity against methicillin-susceptible S. aureus.
h These two combinations would cover most microorganisms, including Streptococcus agalactiae. Gentamicin (or amikacin, in certain cases) could be better for hospital-acquired gram-negative bacteria.
In areas with a high prevalence of MRSA, this group of experts recommends using clindamycin, combined with a β-lactam antibiotic in children under 5 to cover K. kingae. In all cases, if MRSA infection is suspected or confirmed, rifampicin could be added to the treatment.32 The most suitable options in the event of serious MRSA infection (severe sepsis, septic shock and/or septic pulmonary emboli) are listed in Table 4.14,32–34 The antibiotics most commonly used for OAI in children, both orally and via IV, are set out in Tables 3–5.
Oral antibiotic treatment.
| Empirical oral treatment (without microbiological isolate) | |
|---|---|
| Age | Antibiotic |
| Newbornsa and <3 months | Cefuroxime axetilAlternative: A/C |
| 3 months–5 years | Cefuroxime axetil; cefadroxilb in children >2 yearsAlternative: A/C |
| >5 years | Cefadroxilb or cefuroxime axetil |
| Specific treatment according to microbiological isolated | |
|---|---|
| Microorganism | Antibiotic |
| Methicillin-susceptible S. aureus | Cefadroxilb |
| Methicillin-resistant S. aureus | Clindamycin/ciprofloxacin/TMP–SMXc±rifampicinAlternative: linezolid |
| Hib | Cefuroxime axetilAlternative: A/C |
| GBS, GAS, S. pneumoniae | Amoxicillin |
A/C, amoxicillin/clavulanic acid; GAS, S. pyogenes; GBS, S. agalactiae; Hib, Haemophilus influenzae type b; TMP–SMX, trimethoprim–sulfamethoxazole.
At the time of writing this consensus document cefadroxil suspension had ceased to be marketed in Spain; cefuroxime axetil is a suitable alternative, but also suffers from supply problems, leaving amoxicillin/clavulanic acid in solution or preparation from tablets as the only options (see text). In the event of allergy the oral alternatives of the antibiotics commented on in Table 4 could be considered.
If a microbiological isolate is obtained, the treatment will be adjusted, choosing the antibiotic with the narrowest spectrum.
Hospitalisation and duration of intravenous treatmentChildren with an OAI should remain in hospital for initial empirical IV treatment for a minimum of 2–5 days.2,3,10,14,35 Children younger than 3 months could need a longer duration of IV treatment and those <1 month of age should receive most of the antibiotic treatment by this route.35
The duration of treatment, both IV and in total, should be more prolonged in the case of MRSA or PVL-producing MSSA infection, looking out for possible complications.27,28 This group of experts recommends a minimum of 10–14 days of IV treatment in these cases. The duration of treatment of complicated OAI should be determined on an individual basis.
CRP is very useful for monitoring the response to treatment and evaluating when to switch antibiotic treatment to oral administration.2,18 If the patient progresses well, CRP normalises in 7–10 days and ESR in 3–4 weeks.36 An increase, or lack of decrease, in CRP is a very specific marker for negative progression or complications. In order to switch to oral antibiotherapy and discharge the patient, a decrease of at least 30% in the CRP level, absence of fever for 24–48h and an improvement in the signs and symptoms of the infection should be observed.
Outpatient treatment and follow-upThis group of experts recommends using oral cefadroxil whenever possible, with cefuroxime axetil as a suitable alternative (at the time of writing this consensus document, there is a shortage of cefadroxil oral suspension in Spain, as well as serious problems in the supply of all these antibiotics in oral solution – http://www.aeped.es/comite-medicamentos/noticias/retirada-mercado-antibiotico-cefadroxilo-no-todo-esta-perdido-informe-c – and practically the only remaining option is to use amoxicillin/clavulanic acid or crushed adult tablets). In the case of S. pyogenes or penicillin-susceptible S. pneumoniae, the use of oral amoxicillin is recommended.
For oral treatment of CA-MRSA, clindamycin32 or TMP–SMX37 are suggested, according to susceptibility, combined or not with rifampicin.14,32 There is more clinical experience with clindamycin, although its oral tolerability is poorer. A quinolone could be used as an alternative to these.
If there is no microbiological isolate, treatment should be continued using an antibiotic with a similar spectrum to that used intravenously. In the case of cefazolin or cloxacillin, it would continue with cefadroxil or cefuroxime. Table 5 lists the oral antibiotics recommended in different situations.
Following discharge from the hospital it is advisable to follow-up the patient closely, especially for adherence and adverse effects, with assessment at 5–7 days to confirm favourable clinical evolution and tolerability to the antibiotic.
The total duration of antibiotic treatment should never be <10–14 days in the case of SA and 20 days in the case of AOM. In infections by MRSA or PVL-producing MSSA a minimum of 3–4 and 4–6 weeks is recommended for SA and AOM respectively.32Salmonella infection requires more prolonged treatment (4–6 weeks), especially in children with sickle-cell disease. AOM of the pelvis and spine also require minimum durations of 4 weeks.16
While antibiotic treatment continues, this group of experts recommends performing at least a complete blood count and CRP every 10–14 days to monitor the infection and to check for potential adverse effects, although this should be determined on an individual basis. There was no total consensus on the use of ESR for follow-up. Some authors advocated performing an ESR test, especially before ending treatment; others, however, did not advocate it, given that it falls slowly and a prolonged elevated rate could unnecessarily lengthen treatment without signifying unfavourable evolution or complications.36
Discontinuation of treatment should always be conditional on the disappearance of clinical symptoms and normalisation of CRP.2,3 A visit is recommended for finishing the antibiotic treatment and another a month after the end of treatment. Closer follow-up is advisable in complicated cases, when there is axial or pelvic involvement and in infants >3 months of age.
Adjuvant treatmentNon-steroidal anti-inflammatory drugs are recommendable in the acute phase to relieve pain and fever. It has been demonstrated that early treatment of SA with corticosteroids (2–4 days), at the onset of symptoms, can reduce the symptoms and lead to earlier discharge. However, the only two randomised studies diverged over prevention of sequelae.38,39 Therefore, given the low frequency of sequelae in Spain and the possibility of interfering with the diagnosis of noninfectious arthritis or masking the evolution of the process, this group of experts recommends that corticosteroids should not be used routinely, but should be restricted to confirmed infections with a high degree of inflammation (dexamethasone, 0.2mg/kg/8h).39
Complications and prognosisComplications and/or sequelae of acute OAI in children in Spain, with early diagnosis, range between 5% and 10%. They are more common in MRSA infections and/or in the presence of virulence factors such as PVL, infants <3 months old, SA of the hip and delayed diagnoses.5,13,16,19,40 In countries with scarce resources sequelae can be high (up to 30%).
Acute complicationsComplications of OAI should be identified early.
Local complicationsThe most frequent complication is spread from the primary focus to adjacent tissues, especially in younger children. AOM may develop a subperiosteal abscess, spread to the joint (osteoarthritis) or entail muscular involvement (pyomyositis), especially in pelvic locations, and this is relatively common in the case of MRSA.16 These complications must be suspected in the presence of continued fever, persistently positive blood cultures or sustained high CRP.
A much less frequent but serious complication is the appearance of a deep vein thrombosis (DVT), which is more common in adolescent males with osteomyelitis of the femur or tibia caused by S. aureus, especially by MRSA.28
Systemic complicationsOn rare occasions OAI caused by S. aureus can give rise to severe sepsis, with hypotension and multiorgan involvement, which requires admission to an intensive care unit and can be fatal.27,28 Another uncommon complication associated with DVT in osteomyelites caused by S. aureus is septic pulmonary thromboembolism, with respiratory distress and chest pain, which shows up as nodular images and bilateral cavitations on X-rays.28
SequelaeThe consequences of an inadequately treated OAI can be devastating. The most frequent complication is avascular necrosis of the epiphyses (hip and shoulder), followed by a length discrepancy or angular deformity of the extremities and pathological fractures.16 Articular impingement may induce early degeneration of the joint (loss of mobility and pain).
Some of the factors most commonly associated with sequelae are: delay in beginning antibiotherapy, hip involvement, MRSA infection and NB (61%). The treatment of sequelae must be tailored to the individual.
Table 6 sets out the most significant recommendations of this consensus document with the degree of evidence.
Summary of recommendations and evidence.
| • In most cases of SA, children with recent symptoms will be candidates for drainage via arthrocentesis and antibiotherapy, and it is not essential to perform an arthrotomy (BII), which should be considered after 48–72h or 2–3 punctures and aspirations if the response is not satisfactory (AII) |
| • In arthritis of the shoulder and hip, the decision to perform surgical drainage will depend on how early action is taken, on the laboratory analyses and on the experience of the team responsible for the patient. In many cases it may be sufficient to drain and lavage the joint by arthrocentesis, which may need to be repeated (BII). These patients must be treated where there is a team with expertise in this type of childhood infection. Surgical drainage is always more likely to be necessary in infections by high-virulence microorganisms such as PVL-producing S. aureus (AII). In the case of NB and small infants, given the paucity of evidence, a surgical arthrotomy should be performed in most situations (AIII) |
| • If an arthrotomy is performed, placement of a surgical drain should be considered, especially in the hip and the shoulder, and in the youngest infants, for a maximum of 48–72h (BIII), as well as immobilisation of the joint after surgery to avoid complications, implementing early passive mobilisation (CIII). On this point the group of experts did not reach total consensus, and some members do not recommend placement of a drain or immobilisation in most cases (CC, RM) |
| • If OAI is suspected in a child, IV antibiotic treatment should be initiated as soon as possible (AII), after appropriate collection of microbiological samples, which should always include samples for blood culture (AI). The start of antibiotic treatment should not be delayed beyond 6–12h and the minimum duration of IV administration should be 2–5 days (AII) |
| • Children aged <3 months, and especially those of less than a month, should receive a major part of the antibiotic treatment via IV (AII). The duration of treatment, both IV and in total, should be more prolonged and individualised in the case of MRSA or PVL-producing MSSA infection, with a minimum of 10–14 days IV (AII). Equally, the duration of treatment in the case of complicated OAI may need to be prolonged and should be determined on an individual basis |
| • In all cases, an empirical antibiotic with good activity against MSSA and S. pyogenes should be used (AI). In children aged <5 years it is advisable to use an antibiotic with good activity against K. kingae (AI) and in those children aged <5 years with <3 doses of H. influenzae type b or S. pneumoniae vaccine (especially in those under 2 years old) an antibiotic with good coverage against these microorganisms should be used (AII) |
| • Cefazolin is the initial antibiotic of choice in children aged >2 years. Other options are cloxacillin and clindamycin (the latter especially if the prevalence of MRSA is over 10%) (AII). If clindamycin or cloxacillin is used in patients ≤2–5 years it is advisable to combine it with another antibiotic with good activity against Kingella, normally a β-lactam antibiotic (AII) |
| • In children aged <2 years or with <3 doses of vaccine, treatment with cefuroxime is recommended, and as an alternative, amoxicillin/clavulanic acid. Another option would include cloxacillin combined with cefotaxime (BII). This last alternative would be the one indicated for children aged <3 months (AI) |
| • In the event of serious infection, defined as severe sepsis or septic shock, and/or suspicion of septic pulmonary emboli, the most suitable antibiotic would be vancomycin with or without rifampicin (AII), preferably combined with a β-lactam antibiotic with adequate coverage for MSSA until the microbiological isolate is available. If MRSA is identified there are other alternatives, which could include clindamycin, linezolid or daptomycin, in various combinations, with or without rifampicin (BIII) |
| • The duration of IV treatment should never be <2–5 days (AII) |
| • Non-steroidal anti-inflammatory drugs are recommendable in the acute phase for relief of pain and fever in OAI (AII). The use of corticosteroids is confined to situations with a high degree of inflammation, especially if the infectious aetiology is confirmed or is very probable (BII) |
| • A decrease of at least 30% in CRP levels with absence of fever for ≥24–48h and improvement in the signs and symptoms of the infection allow oral antibiotic treatment to be initiated and discharge from hospital to be considered (AII) |
| • Evolution of OAI with antibiotic treatment should be positive. A lack of response to antibiotic treatment indicates a resistant pathogen, a developing complication or a non-infectious diagnosis (BII) |
| • This panel recommends the use of oral cefadroxil in Spain, whenever possible, in view of its good tolerability, narrow spectrum and substantial clinical experience (AII). Cefuroxime axetil or amoxicillin/clavulanic acid are alternatives, if there is a shortage. In the case of S. pyogenes or S. pneumoniae with good susceptibility to penicillin, oral amoxicillin is recommended (AII) |
| • For oral treatment of community-acquired MRSA, this group of experts recommends the use of clindamycin (AII), TMP–SMX (BII) or ciprofloxacin (BII), combined or not with rifampicin (CIII). Treatment with quinolones in monotherapy should be avoided. |
| • If no microbe is isolated, treatment should be continued using an antibiotic with a similar spectrum to that used intravenously (AII) |
| • It is advisable to give the patient an appointment at the outpatient clinic in 5–7 days (BIII) |
| • The total duration of antibiotic treatment should never be <10–14 days in the case of SA (AII) and 20 days in the case of AOM (AII). Discontinuation of treatment should always be conditional on the disappearance of clinical symptoms and normalisation of CRP (AII) |
| • More prolonged follow-up of NB and small infants, hip involvement and complicated OAI should be undertaken by orthopaedics and/or rheumatology (AIII) |
The authors have no conflicts of interest to declare.
To the SERPE Osteoarticular Infections Group.
Please cite this article as: Saavedra-Lozano J, Calvo C, Huguet Carol R, Rodrigo C, Núñez E, Obando I, et al. Documento de consenso SEIP-SERPE-SEOP sobre el tratamiento de la osteomielitis aguda y artritis séptica no complicadas. An Pediatr (Barc). 2015;82:273.e1–273.e10.