To evaluate the impact of the sedation-analgesia technique on the pain experienced by the patient.
MethodsThis cross-sectional study was conducted on consecutive patients with cerebral palsy (CP) who underwent infiltration with botulinum toxin A (BoTNA). The patients were divided into 4 different groups according to the analgesic strategy assigned: Group I, without sedation or topical anaesthetic cream; Group II, inhalation of nitrous oxide; Group III, deep intravenous sedation; and Group IV, light sedation with benzodiazepines. Pain was assessed with different scales depending on patient age. Parents were asked to rate their satisfaction with their child's comfort by using a 5-point Likert-type scale. The primary end-point was the proportion of patients that experienced a pain level equal or lower than 2, according to pain scales, in the different study groups.
ResultsOf the 124 patients included in the study, 56 (45.2%) experienced a pain level≤2. In the Group III a significantly greater proportion of patients were classified with a pain level score≤2, P<.001, as compared with all the study groups, respectively. The BoTNA injection was guided by ultrasonography in 109 (87.9%) patients, and by palpation in 15 (12.1%).
ConclusionThe results of this study suggested that, in patients with CP treated with BoTNA injections, the sedation-analgesic strategy had a significant impact on the pain experienced by the subject. Selecting an appropriate analgesic strategy is crucial for reducing the stress associated with the administration of BoTNA injections in children with CP.
Evaluar el impacto de la técnica sedoanalgésica en el dolor experimentado por el paciente.
MétodosEstudio transversal realizado en pacientes consecutivos con parálisis cerebral (PC) que se infiltraron con toxina botulínica A (OnabotA). Los pacientes fueron divididos en 4 grupos según la estrategia analgésica asignada: Grupo I, sin sedación o crema anestésica tópica; Grupo II, inhalación de óxido nitroso; Grupo III, sedación intravenosa profunda y Grupo IV, sedación ligera con benzodiacepinas. El dolor se evaluó con diferentes escalas según la edad del paciente. Los padres clasificaron su satisfacción con la comodidad de su hijo mediante una escala tipo Likert de 5 puntos. La variable primaria de eficacia fue la proporción de pacientes que experimentaron un nivel de dolor ≤2, según las escalas de dolor, en los diferentes grupos de estudio.
ResultadosDe los 124 pacientes incluidos en el estudio, 56 (45,2%) experimentaron un nivel de dolor ≤2. En el Grupo III, una proporción significativamente mayor de pacientes presentó un nivel de dolor ≤2, p<0,001, en comparación con todos los grupos de estudio, respectivamente. La inyección de OnabotA fue guiada por ultrasonografía en 109 (87,9%) pacientes y por referencia anatómica en 15 (12,1%).
ConclusiónEn los pacientes con PC tratados con infiltraciones de OnabotA, la estrategia sedoanalgésica tuvo un impacto significativo en el dolor experimentado por el sujeto. Seleccionar una estrategia analgésica apropiada es crucial para reducir el estrés asociado con la administración de inyecciones de OnabotA en niños con PC.
Cerebral palsy (CP) is a heterogeneous group of nonprogressive permanent diseases of posture and movement control.1,2 In preterm and full-term newborns alike, it is associated with several prenatal, perinatal and postnatal factors, such as preeclampsia and intrauterine growth restriction, preterm birth, low birth weight, infection/inflammation, multiple pregnancy, other complications of pregnancy, respiratory distress and genetic disorders.2–5 The prevalence of CP ranges between 2 and 2.5 cases per 1000 live births2,6 and its incidence may be increasing due to improvements in obstetric and neonatal care.4,7 Different classifications of CP have been proposed based on the location and clinical manifestations of the brain lesions.8–11 Thus, CP may be classified according to a topographical approach or a physiological approach.12
Approximately 80% of children with CP will present with spasticity as the main motor impairment.12 Spasticity can be defined as velocity-dependent hypertonia associated with an increased myotatic reflex and is part of upper motor neuron syndrome.13
One of the most common methods used to assess CP in children is the expanded and revised Gross Motor Function Classification System (GMFCS).14,15
Due to the heterogeneous nature of CP, each patient needs to be managed with an individualised approach. Among the various nonsurgical treatment options, the use of botulinum toxin type A (BoNTA) is recommended in standard clinical practice (level A recommendation).5,16–18 Injection of BoNTA has been recommended as a therapeutic option in the treatment of dynamic equinovarus foot in children with CP, for whom no other conservative treatment has proven more effective (Level A recommendation).16
The efficacy and safety of the use of BoNTA for management of spasticity in the upper extremities in children with CP have been established in previous studies.19,20 In these patients, the use of BoNTA must be combined with occupational therapy programmes (strong recommendation, green light intervention).18 Several studies have demonstrated the efficacy of BoNTA in the treatment of spasticity in the lower extremities, with improvements in deformity and gait.21–23
From the perspective of specific goals, BoNTA is used for a broad range of indications, such as facilitating stretches in physical therapy and management of pain associated with spasms, including postoperative pain.24
Two main aspects must be taken into account when administering BoNTA to children: the technique to guide the injection and the sedation and analgesia strategy. Several techniques are available to guide the injection, including palpation, ultrasound, electromyography (EMG) or electrical stimulation.24
The selection of an adequate sedation and analgesia protocol (SAP) is necessary to reduce pain and anxiety in children that receive intramuscular BoNTA injections.
There is evidence of the efficacy and adequate tolerability of inhalation of a mix of oxygen and nitrous oxide for sedation and analgesia during intramuscular injection of BoNTA in children.25 However, an analgesia protocol consisting of nitrous oxide and anaesthetic cream was only effective in 50% of children.26
The development of a standard procedural SAP could help reduce pain and eliminate the anxiety resulting from these injections.
The aim of our study was to assess the impact of the sedation and analgesia strategy in the pain experienced by the patient.
MethodsWe conducted a cross-sectional study in patients with CP managed in the Paediatric Rehabilitation Unit of the Hospital Materno Infantil in the city of Malaga, which we included by consecutive sampling according to the established inclusion and exclusion criteria, that were treated with BoNTA between November 2015 and June 2016.
The study protocol was approved by the Ethics Committee of the hospital. The parents of every participant received detailed information about the characteristics of the study and gave their consent in writing. We conducted the study in adherence with the ethical principles established in the Declaration of Helsinki and with good clinical practice guidelines.
The inclusion criteria were the following: children/adolescent aged 2–16 years, scheduled to receive BoNTA injections for treatment of spasticity. We excluded patients if they had any contraindication for treatment with BoNTA (history of allergic reaction, neuromuscular junction disorder, peripheral neuropathy, etc.), a coagulation disorder, could not tolerate sedation or were unwilling to comply with the protocol or the directions given by the researcher.
Study groupsWe assigned patients to 1 of 4 groups based on the SAP. Group I included 2 subsets, the patients that did not receive any sedation and the patients that had a topical anaesthetic cream applied to the planned injection sites at least 30min before the procedure; Group II received inhaled nitrous oxide under the supervision of a paediatrician; Group III intravenous deep sedation under the supervision of an anaesthesiologist; Group IV mild sedation with oral or rectal administration of benzodiazepines.
Cooperative patients (defined as those capable to maintain of change their posture to facilitate a precise location of injection of BoNTA) who required fewer than 4 injections were assigned to Group I. Groups II and IV included patients that had exhibited poor cooperation in previous injections precluding the correct administration of BoNTA as well as patients that required more than 4 injections. Group III included patients with poor cooperation in previous injections (defined in the same way as in Group II), and those who required more than 8 injections or injection in deep muscles.
Level of sedationThe level of sedation was selected based on the injection sites (number of injections and depth of intramuscular injection), the level of cooperation of the child and the available resources.
Assessment of severity of spasticityWe used the GMFCS and the modified Ashworth scale (MAS) to assess the severity of spasticity. The classification of severity by means of the GMFCS offers descriptions of changes in gross motor functioning by age group within each severity level.14,15 This method classifies functional impairment on a 5-level scale. Level I includes patients that can walk without restrictions; level II patients that walk with certain limitations; level III patients that walk using a handheld mobility device; level IV patients with limited autonomous mobility, possibly using powered mobility; and level patients who are always transported in a wheelchair.14,15
The MAS is probably the most widely used method to measure spasticity in clinical practice and research.27 It is a 6-point scale that classifies the resistance of a relaxed extremity to quick passive stretching.27
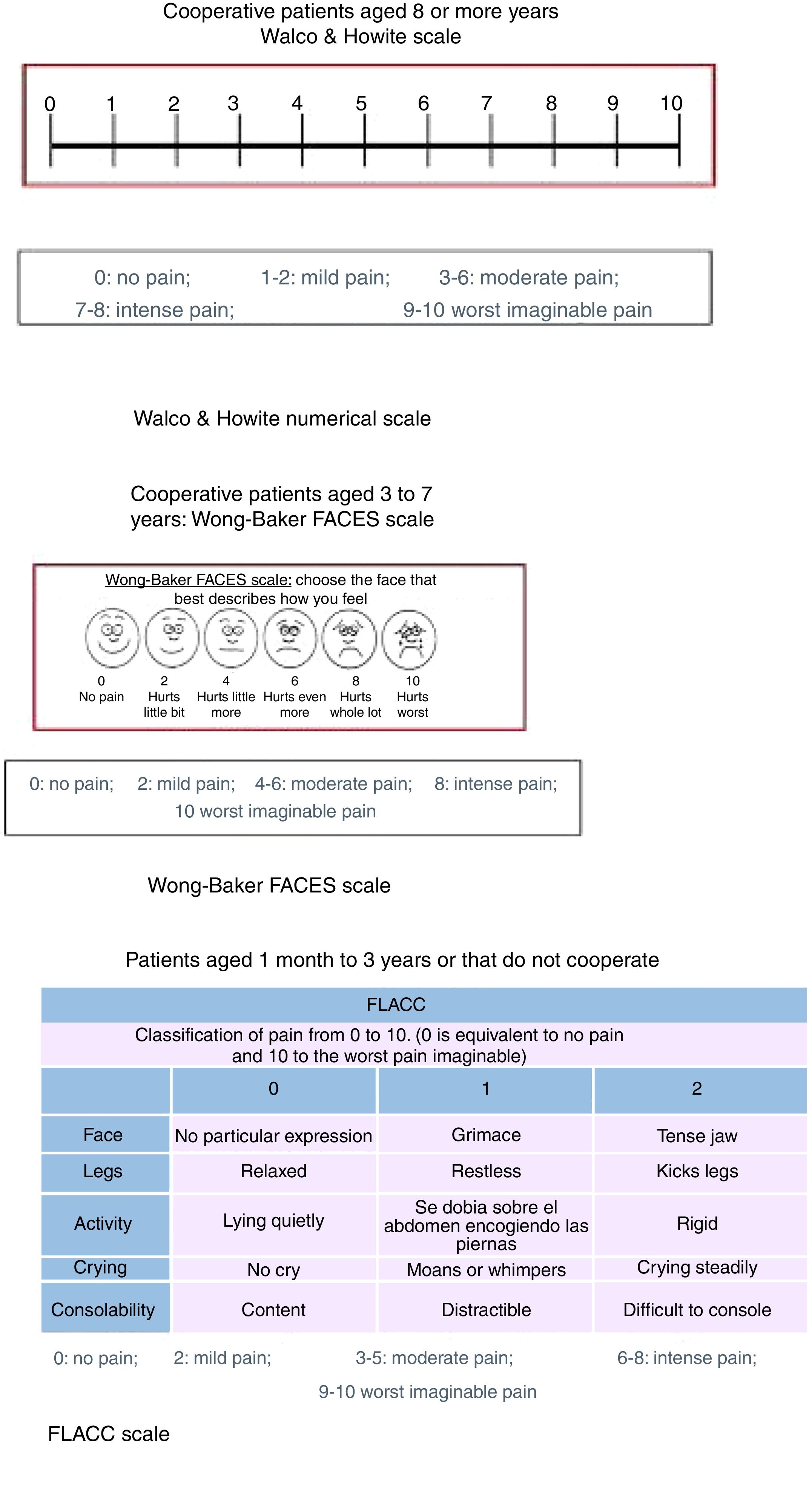
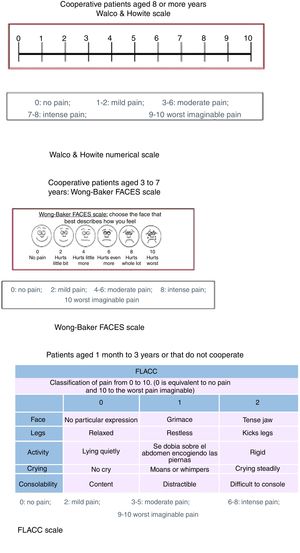
Assessment of painPatients aged less than 3 yearsA trained observer assessed pain by means of the Face, Legs, Activity, Cry, Consolability (FLACC), a validated instrument with a possible score ranging from 0 to 10. It is based on the observation of facial expressions, leg movements, the level of activity, crying and ability to be soothed. Higher scores are indicative of higher levels of discomfort.28
Patients aged 3–7 yearsWe assessed pain by means of the Wong-Baker FACES pain rating scale (WBS), one of the existing faces scales that has been proven useful in multiple paediatric settings for assessment of pain.29 Patients were instructed to circle the face that best represented their level of pain. After this, we asked patients to rate their pain on a visual analogue scale (VAS) measuring 100mm with a horizontal orientation whose extremes were marked as “no pain” and “worst imaginable pain”.
Patients aged 8 or more yearsWe used a visual numerical scale developed by Walco and Howite with which pain is assessed as follows: 0, no pain; 1–2, mild pain; 3–6, moderate pain, 7–8, severe pain grave and 9–10, the most pain I’ve ever felt.
Fig. 1 shows the results of the MAS, numerical Walco and Howite pain scale, WBS and FLACC scale.
Results of the modified Ashworth scale, numerical pain scale of Walco and Howite, Wong-Baker FACES scale and FLACC scale. FLACC, Face, Legs, Activity, Crying and Consolability. Modified Ashworth scale: Subjective scale that rates the severity of spasticity from 0 to 4, of proven reliability in adults (in its modified version) for assessment of spasticity in the elbow flexors and plantar flexors. Values: 0 No increase in muscle tone. 1 Slight increase in muscle tone, manifested by minimal resistance in part of the joint range of motion. 1+ Presence of a catch at some point in the range of motion. 2 More marked increase in muscle tone through most of the range of motion. 3 Considerable increase in muscle tone, passive movement difficult. 4 Rigid muscle contraction.
We asked parents to rate their satisfaction with the wellbeing of their children in relation to the assigned analgesia protocol through a questionnaire that used a 5-point Likert scale (1, strongly disagree; 2, disagree; 3, neither agree nor disagree; 4, agree; 5, strongly agree). The questionnaire consisted of the following items: [1] I think that my child experienced pain; [2] I think that the level of cooperation of my child was adequate; [3] The injection of BoNTA was stressful to my child; [4] I think my child will not consider this a traumatic experience.
Satisfaction of health care professionals with sedationWe asked physicians to rate their level of satisfaction using 5-point Likert scales (similar to the scales used in parents). A single item was assessed: [1] The cooperation of the patient allowed me to perform the intervention.
Variables under studyThe variables under study were age, sex, SAP, injection method (using anatomical references or ultrasound), type of CP (spastic, dyskinetic, ataxic and mixed), functional classification of CP, GMFCS score, MAS score, number of injections, total dose of BoNTA, number of injected muscles, level of pain (based on the different scales), and parental satisfaction and physician satisfaction with the selected SAP.
Primary outcome measureThe proportion of patients that experienced a level of pain equal or inferior to 2, based on the pain scales, in the different study groups.
Secondary outcome measuresOne of the secondary effectiveness outcome measures was the difference in the level of pain between the different SAPs. Other secondary outcomes were the associations between the level of pain and different variables under study (GMFCS score, dose of BoNTA, number of injections, MAS score, functional classification of CP and age) and the level of satisfaction of parents and physicians with the SAP.
Statistical analysisWe performed the analyses with the statistical software MedCalc version 18 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2018).
Before starting the study, we determined that we needed a minimum of 28 participants per study group to detect a difference equal to or greater than 21% between groups, for an alpha error probability of 0.05 and a beta error probability of 0.2. We have summarised the data as absolute frequencies and percentages, mean and standard deviation (SD), mean and 95% confidence interval (95% CI) or median and 95% CI, as applicable.
We assessed the normality of the distribution of the data by means of the d’Agostino-Pearson test. In case of a normal distribution of quantitative data, we used one-way analysis of variance (ANOVA) to compare the means of different groups. We used analysis of covariance (ANCOVA) to compare the mean pain scores between different SAPs setting the age, number of injected muscles and number of injections as covariates.
For quantitative variables that did not follow a normal distribution, we made comparisons by means of the Kruskal–Wallis test. We compared categorical data using the chi-square test or the Fisher exact test as applicable.
To assess the association between pain and the different variables under study (GMFCS score, dose of BoNTA, number of injections, MAS score and age), we used linear regression and the Pearson correlation coefficient (r).
We defined statistical significance as a P-value of less than 0.05.
ResultsA total of 124 patients met the inclusion criteria in absence of exclusion criteria: 32 (Group I); 33 (Group II); 29 (Group III) and 30 (Group IV).
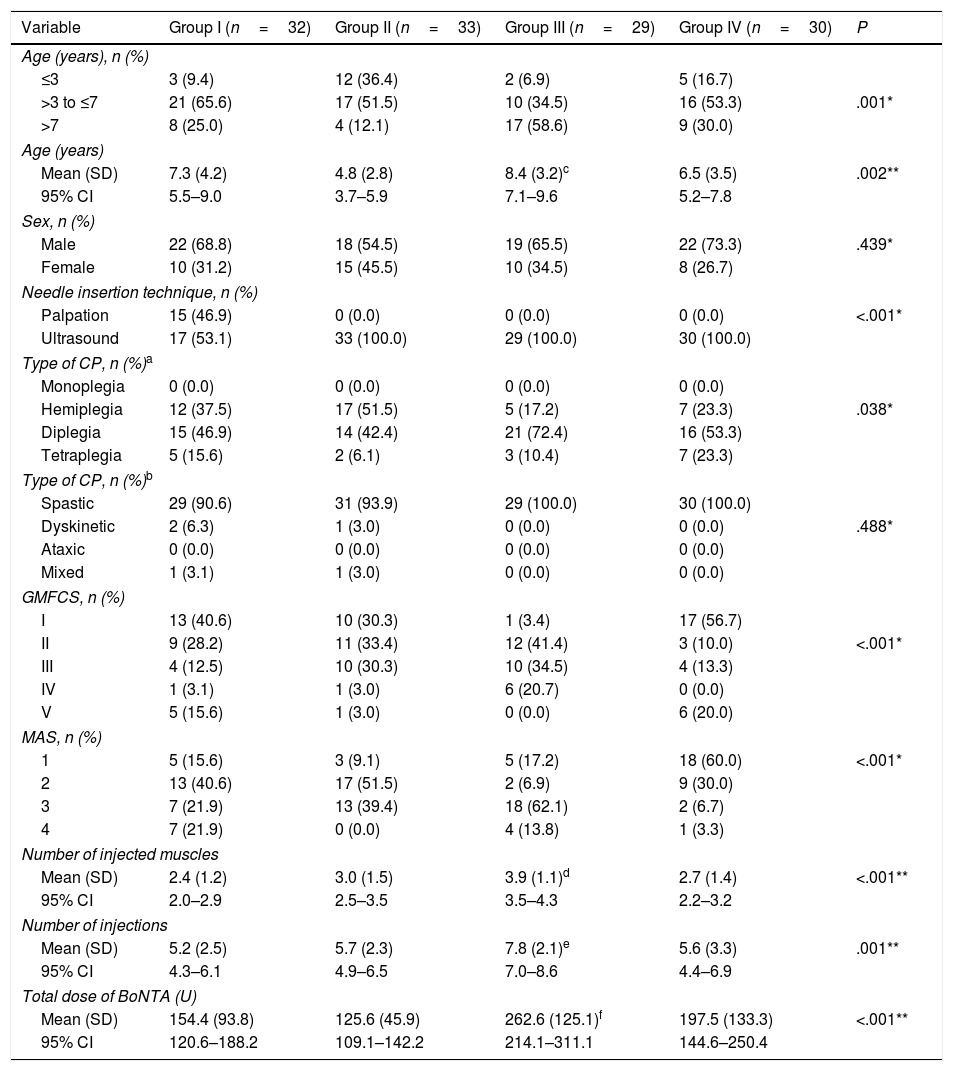
Table 1 presents the main demographic and clinical characteristics of the sample.
Demographic and clinical characteristics of the patients included in the study.
| Variable | Group I (n=32) | Group II (n=33) | Group III (n=29) | Group IV (n=30) | P |
|---|---|---|---|---|---|
| Age (years), n (%) | |||||
| ≤3 | 3 (9.4) | 12 (36.4) | 2 (6.9) | 5 (16.7) | |
| >3 to ≤7 | 21 (65.6) | 17 (51.5) | 10 (34.5) | 16 (53.3) | .001* |
| >7 | 8 (25.0) | 4 (12.1) | 17 (58.6) | 9 (30.0) | |
| Age (years) | |||||
| Mean (SD) | 7.3 (4.2) | 4.8 (2.8) | 8.4 (3.2)c | 6.5 (3.5) | .002** |
| 95% CI | 5.5–9.0 | 3.7–5.9 | 7.1–9.6 | 5.2–7.8 | |
| Sex, n (%) | |||||
| Male | 22 (68.8) | 18 (54.5) | 19 (65.5) | 22 (73.3) | .439* |
| Female | 10 (31.2) | 15 (45.5) | 10 (34.5) | 8 (26.7) | |
| Needle insertion technique, n (%) | |||||
| Palpation | 15 (46.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <.001* |
| Ultrasound | 17 (53.1) | 33 (100.0) | 29 (100.0) | 30 (100.0) | |
| Type of CP, n (%)a | |||||
| Monoplegia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hemiplegia | 12 (37.5) | 17 (51.5) | 5 (17.2) | 7 (23.3) | .038* |
| Diplegia | 15 (46.9) | 14 (42.4) | 21 (72.4) | 16 (53.3) | |
| Tetraplegia | 5 (15.6) | 2 (6.1) | 3 (10.4) | 7 (23.3) | |
| Type of CP, n (%)b | |||||
| Spastic | 29 (90.6) | 31 (93.9) | 29 (100.0) | 30 (100.0) | |
| Dyskinetic | 2 (6.3) | 1 (3.0) | 0 (0.0) | 0 (0.0) | .488* |
| Ataxic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Mixed | 1 (3.1) | 1 (3.0) | 0 (0.0) | 0 (0.0) | |
| GMFCS, n (%) | |||||
| I | 13 (40.6) | 10 (30.3) | 1 (3.4) | 17 (56.7) | |
| II | 9 (28.2) | 11 (33.4) | 12 (41.4) | 3 (10.0) | <.001* |
| III | 4 (12.5) | 10 (30.3) | 10 (34.5) | 4 (13.3) | |
| IV | 1 (3.1) | 1 (3.0) | 6 (20.7) | 0 (0.0) | |
| V | 5 (15.6) | 1 (3.0) | 0 (0.0) | 6 (20.0) | |
| MAS, n (%) | |||||
| 1 | 5 (15.6) | 3 (9.1) | 5 (17.2) | 18 (60.0) | <.001* |
| 2 | 13 (40.6) | 17 (51.5) | 2 (6.9) | 9 (30.0) | |
| 3 | 7 (21.9) | 13 (39.4) | 18 (62.1) | 2 (6.7) | |
| 4 | 7 (21.9) | 0 (0.0) | 4 (13.8) | 1 (3.3) | |
| Number of injected muscles | |||||
| Mean (SD) | 2.4 (1.2) | 3.0 (1.5) | 3.9 (1.1)d | 2.7 (1.4) | <.001** |
| 95% CI | 2.0–2.9 | 2.5–3.5 | 3.5–4.3 | 2.2–3.2 | |
| Number of injections | |||||
| Mean (SD) | 5.2 (2.5) | 5.7 (2.3) | 7.8 (2.1)e | 5.6 (3.3) | .001** |
| 95% CI | 4.3–6.1 | 4.9–6.5 | 7.0–8.6 | 4.4–6.9 | |
| Total dose of BoNTA (U) | |||||
| Mean (SD) | 154.4 (93.8) | 125.6 (45.9) | 262.6 (125.1)f | 197.5 (133.3) | <.001** |
| 95% CI | 120.6–188.2 | 109.1–142.2 | 214.1–311.1 | 144.6–250.4 | |
BoNTA, botulinum toxin type A; CI, confidence interval; CP, cerebral palsy; GMFCS, Gross Motor Function Classification System; MAS, Modified Ashworth Scale; SD, standard deviation.
The number of injections ranged between 2 and 14 and the number of injected muscles between 1 and 7.
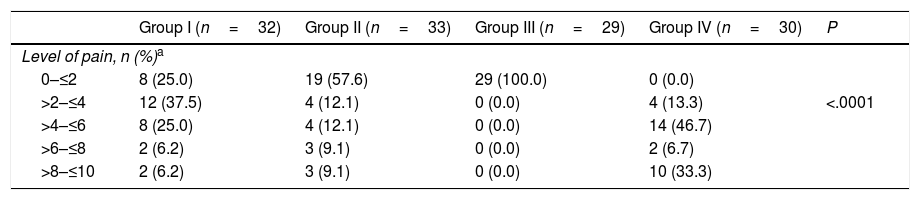
Of the 124 participants, 56 (45.2%) had a level of pain equal to or less than 2 (Table 2).
Proportion of patients in each group under study that experienced a specific level of pain.
| Group I (n=32) | Group II (n=33) | Group III (n=29) | Group IV (n=30) | P | |
|---|---|---|---|---|---|
| Level of pain, n (%)a | |||||
| 0–≤2 | 8 (25.0) | 19 (57.6) | 29 (100.0) | 0 (0.0) | |
| >2–≤4 | 12 (37.5) | 4 (12.1) | 0 (0.0) | 4 (13.3) | <.0001 |
| >4–≤6 | 8 (25.0) | 4 (12.1) | 0 (0.0) | 14 (46.7) | |
| >6–≤8 | 2 (6.2) | 3 (9.1) | 0 (0.0) | 2 (6.7) | |
| >8–≤10 | 2 (6.2) | 3 (9.1) | 0 (0.0) | 10 (33.3) | |
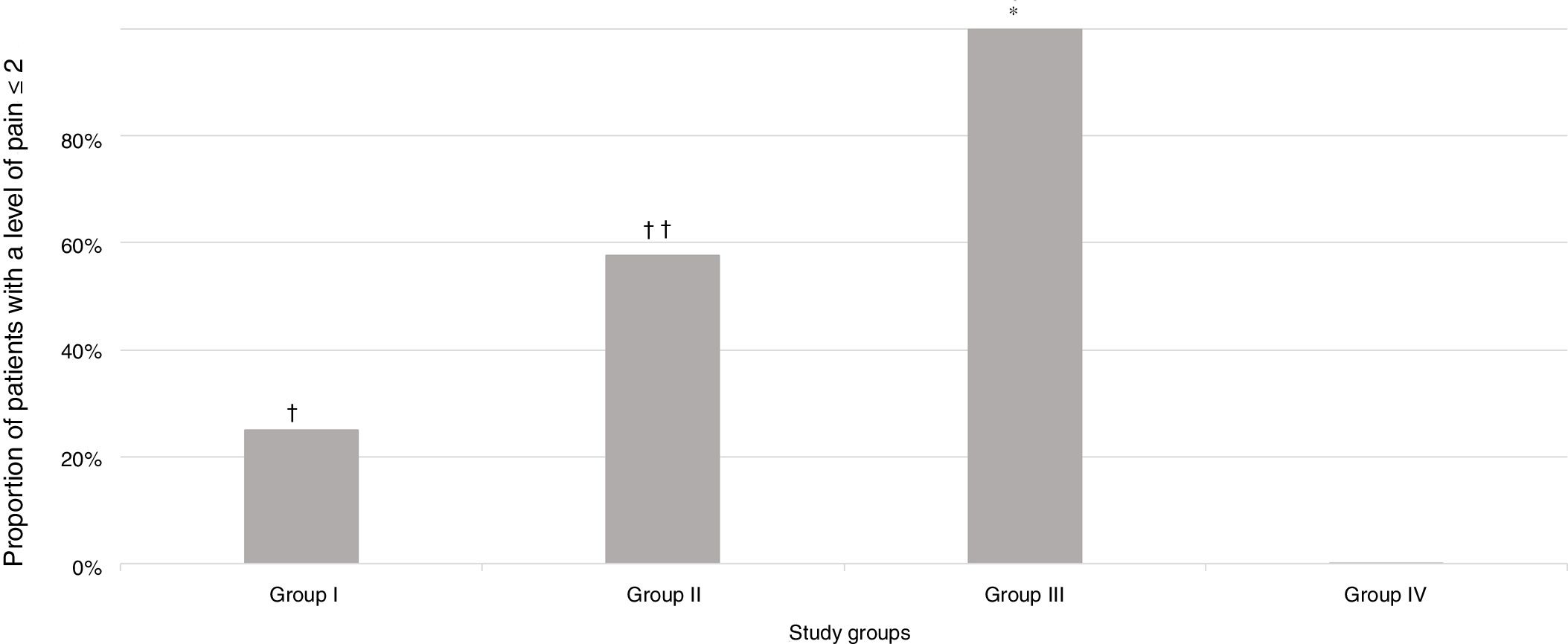
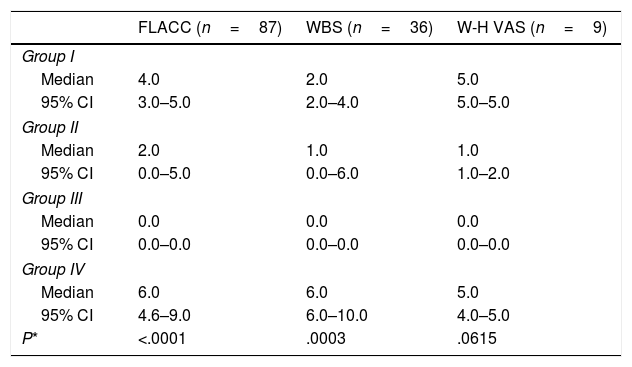
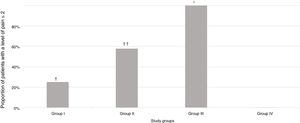
A significantly greater proportion of patients had a pain score of 2 or less in Group III (intravenous deep sedation under the supervision of an anaesthesiologist) compared to all other groups under study (P<.001) (Fig. 2).
Proportion of patients in each group under study that experienced a level of pain of 2 or less based on the pain scale scores. † P=.005 in comparison with the group under study. †† P=.01 in comparison with Group I and .001 in comparison with Group IV. * P<.001 in comparison with all groups under study.
The injection of BoNTA was guided by ultrasound in 109 patients (87.9%) and by anatomical localisation in 15 (12.1%). Since manual injection based on anatomical landmarks was only used in Group I, we compared the level of pain within this group in patients that received ultrasound-guided BoNTA injections versus patients that received injections with insertion based on anatomical landmarks. The mean pain score was 3.36 (SD, 0.76) in patients in whom needle insertion was guided by anatomical landmarks and 5.19 (0.76) in patients in which it was guided by ultrasound (P=.1193).
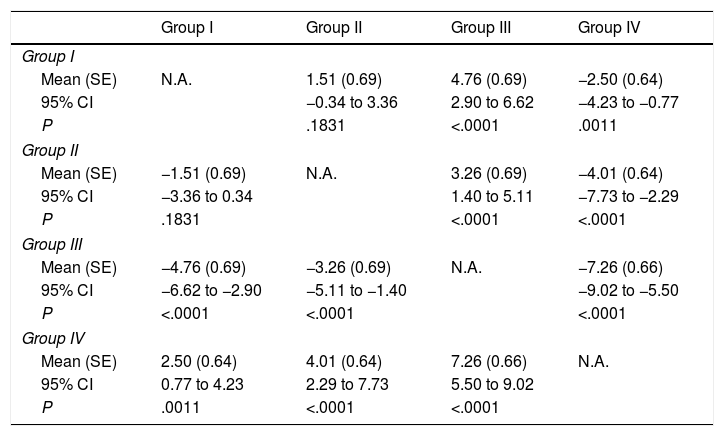
Pain during administration of BoNTA was significantly lesser in children that received intravenous deep sedation under the supervision of an anaesthesiologist compared to children managed with other sedation strategies (Tables 3 and 4).
Comparison of the level of pain assessed with the different scales used in the study based on the sedation and analgesia protocol.
| FLACC (n=87) | WBS (n=36) | W-H VAS (n=9) | |
|---|---|---|---|
| Group I | |||
| Median | 4.0 | 2.0 | 5.0 |
| 95% CI | 3.0–5.0 | 2.0–4.0 | 5.0–5.0 |
| Group II | |||
| Median | 2.0 | 1.0 | 1.0 |
| 95% CI | 0.0–5.0 | 0.0–6.0 | 1.0–2.0 |
| Group III | |||
| Median | 0.0 | 0.0 | 0.0 |
| 95% CI | 0.0–0.0 | 0.0–0.0 | 0.0–0.0 |
| Group IV | |||
| Median | 6.0 | 6.0 | 5.0 |
| 95% CI | 4.6–9.0 | 6.0–10.0 | 4.0–5.0 |
| P* | <.0001 | .0003 | .0615 |
The number of tests is greater than 124 because 8 participants were assessed with both the FLACC and the WBS scales.
CI, confidence interval; FLACC, Face, Legs, Activity, Cry, Consolability scale; WBS, Wong-Baker FACES scale; W-H VAS, Walco & Howite visual analogue scale.
Mean difference in perceived pain between the groups under study.
| Group I | Group II | Group III | Group IV | |
|---|---|---|---|---|
| Group I | ||||
| Mean (SE) | N.A. | 1.51 (0.69) | 4.76 (0.69) | −2.50 (0.64) |
| 95% CI | −0.34 to 3.36 | 2.90 to 6.62 | −4.23 to −0.77 | |
| P | .1831 | <.0001 | .0011 | |
| Group II | ||||
| Mean (SE) | −1.51 (0.69) | N.A. | 3.26 (0.69) | −4.01 (0.64) |
| 95% CI | −3.36 to 0.34 | 1.40 to 5.11 | −7.73 to −2.29 | |
| P | .1831 | <.0001 | <.0001 | |
| Group III | ||||
| Mean (SE) | −4.76 (0.69) | −3.26 (0.69) | N.A. | −7.26 (0.66) |
| 95% CI | −6.62 to −2.90 | −5.11 to −1.40 | −9.02 to −5.50 | |
| P | <.0001 | <.0001 | <.0001 | |
| Group IV | ||||
| Mean (SE) | 2.50 (0.64) | 4.01 (0.64) | 7.26 (0.66) | N.A. |
| 95% CI | 0.77 to 4.23 | 2.29 to 7.73 | 5.50 to 9.02 | |
| P | .0011 | <.0001 | <.0001 | |
We obtained the P-values for paired comparisons by means of analysis of covariance (ANCOVA), with age, the number of injections and the number of injected muscles as covariates.
CI, confidence interval; N.A., not applicable; SE, standard error.
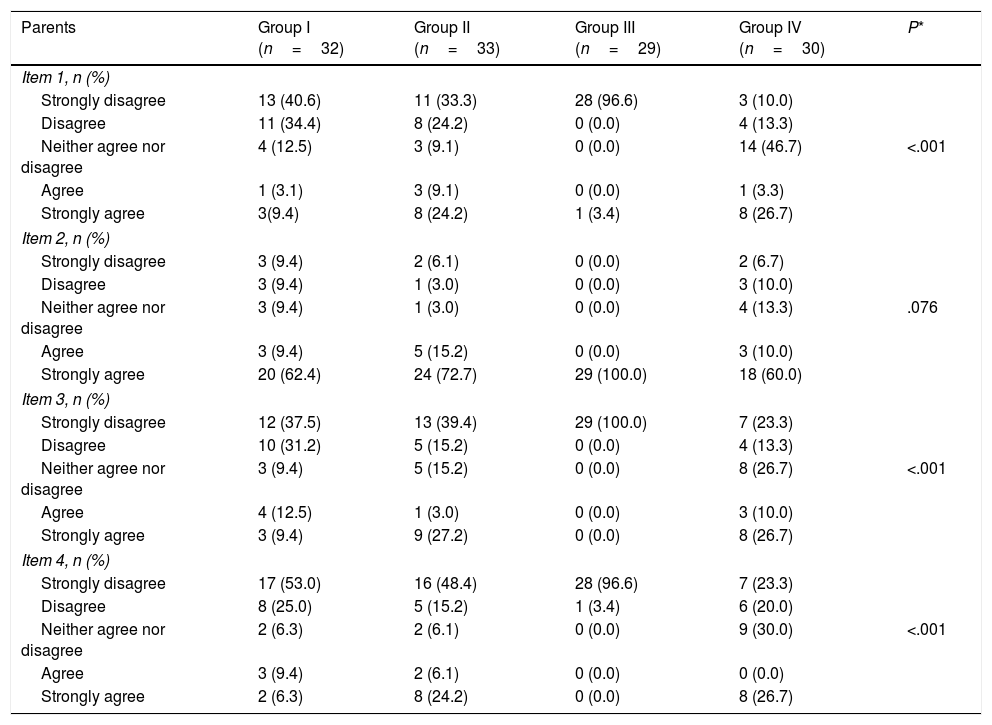
When it came to parental satisfaction, the proportion of parents that “strongly agreed” or “agreed” with the item “I think that the level of cooperation of my child was adequate” ranged between 70% in Group IV and 100% in Group III (P=.076) (Table 5). However, we found statistically significant differences in the other items (Table 5).
Comparison of the satisfaction of parents and health professionals with sedation by study group.
| Parents | Group I (n=32) | Group II (n=33) | Group III (n=29) | Group IV (n=30) | P* |
|---|---|---|---|---|---|
| Item 1, n (%) | |||||
| Strongly disagree | 13 (40.6) | 11 (33.3) | 28 (96.6) | 3 (10.0) | |
| Disagree | 11 (34.4) | 8 (24.2) | 0 (0.0) | 4 (13.3) | |
| Neither agree nor disagree | 4 (12.5) | 3 (9.1) | 0 (0.0) | 14 (46.7) | <.001 |
| Agree | 1 (3.1) | 3 (9.1) | 0 (0.0) | 1 (3.3) | |
| Strongly agree | 3(9.4) | 8 (24.2) | 1 (3.4) | 8 (26.7) | |
| Item 2, n (%) | |||||
| Strongly disagree | 3 (9.4) | 2 (6.1) | 0 (0.0) | 2 (6.7) | |
| Disagree | 3 (9.4) | 1 (3.0) | 0 (0.0) | 3 (10.0) | |
| Neither agree nor disagree | 3 (9.4) | 1 (3.0) | 0 (0.0) | 4 (13.3) | .076 |
| Agree | 3 (9.4) | 5 (15.2) | 0 (0.0) | 3 (10.0) | |
| Strongly agree | 20 (62.4) | 24 (72.7) | 29 (100.0) | 18 (60.0) | |
| Item 3, n (%) | |||||
| Strongly disagree | 12 (37.5) | 13 (39.4) | 29 (100.0) | 7 (23.3) | |
| Disagree | 10 (31.2) | 5 (15.2) | 0 (0.0) | 4 (13.3) | |
| Neither agree nor disagree | 3 (9.4) | 5 (15.2) | 0 (0.0) | 8 (26.7) | <.001 |
| Agree | 4 (12.5) | 1 (3.0) | 0 (0.0) | 3 (10.0) | |
| Strongly agree | 3 (9.4) | 9 (27.2) | 0 (0.0) | 8 (26.7) | |
| Item 4, n (%) | |||||
| Strongly disagree | 17 (53.0) | 16 (48.4) | 28 (96.6) | 7 (23.3) | |
| Disagree | 8 (25.0) | 5 (15.2) | 1 (3.4) | 6 (20.0) | |
| Neither agree nor disagree | 2 (6.3) | 2 (6.1) | 0 (0.0) | 9 (30.0) | <.001 |
| Agree | 3 (9.4) | 2 (6.1) | 0 (0.0) | 0 (0.0) | |
| Strongly agree | 2 (6.3) | 8 (24.2) | 0 (0.0) | 8 (26.7) | |
| Health professionals | Group I | Group II | Group III | Group IV | P |
|---|---|---|---|---|---|
| Question 1, n (%) | |||||
| Strongly disagree | 1 (3.1) | 3 (9.1) | 0 (0.0) | 5 (16.7) | |
| Disagree | 1 (3.1) | 0 (0.0) | 0 (0.0) | 4 (13.3) | |
| Neither agree nor disagree | 3 (9.4) | 2 (6.1) | 0 (0.0) | 0 (0.0) | 0.005 |
| Agree | 5 (15.6) | 4 (12.1) | 0 (0.0) | 3 (10.0) | |
| Strongly agree | 22 (68.8) | 24 (72.7) | 29 (100.0) | 18 (60.0) | |
Item 1: I think that my child I think that the level of cooperation of my child was adequate; Item 3: The injection of BoNTA was stressful to my child; Item 4: I think my child will not consider this a traumatic experience; Question 1: The cooperation of the patient allowed me to perform the intervention.
The proportion of physicians that “agreed” or “strongly agreed” with the statement “The cooperation of the patient allowed me to perform the intervention” ranged between 70% for Group IV and 100 for Group V (Table 5).
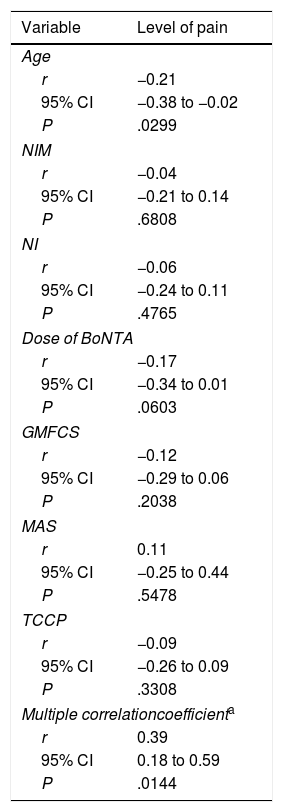
Table 6 presents the correlation between the level of pain and some of the variables under study. Pain was significantly correlated to age (r=−0.21; P=.0299) (Table 6).
Correlation between pain (assessed with different methods) and some of the variables under study (age, number of injected muscles, number of injections, Gross Motor Function Classification System, Modified Ashworth Scale and topographical classification of cerebral palsy).
| Variable | Level of pain |
|---|---|
| Age | |
| r | −0.21 |
| 95% CI | −0.38 to −0.02 |
| P | .0299 |
| NIM | |
| r | −0.04 |
| 95% CI | −0.21 to 0.14 |
| P | .6808 |
| NI | |
| r | −0.06 |
| 95% CI | −0.24 to 0.11 |
| P | .4765 |
| Dose of BoNTA | |
| r | −0.17 |
| 95% CI | −0.34 to 0.01 |
| P | .0603 |
| GMFCS | |
| r | −0.12 |
| 95% CI | −0.29 to 0.06 |
| P | .2038 |
| MAS | |
| r | 0.11 |
| 95% CI | −0.25 to 0.44 |
| P | .5478 |
| TCCP | |
| r | −0.09 |
| 95% CI | −0.26 to 0.09 |
| P | .3308 |
| Multiple correlationcoefficienta | |
| r | 0.39 |
| 95% CI | 0.18 to 0.59 |
| P | .0144 |
BoNTA, botulinum toxin type A; CI, confidence interval; GMFCS, Gross Motor Function Classification System; MAS, Modified Ashworth Scale; NI, number of injections; NIM, number of injected muscles; r, Pearson correlation coefficient; TCCP, topographical classification of cerebral palsy.
The results of our study show that the choice of sedation and analgesia protocol has a significant impact in the level of pain experienced by patients with CP treated with BoNTA.
Although the response to children to stressful or painful situations differs substantially between individuals,30 there is evidence in the literature that inadequate pain management in children has a negative impact on future experiences involving painful processes.25,31 The recollection of a painful medical procedure can reduce the effect of pain medication in subsequent procedures31; thus, one study found that the memory of a painful dental procedure in the past was a predictor for reported pain in a subsequent dental procedure.32
In children that require repeated painful procedures, the pain experienced during the initial procedure has a significant influence on pain and anxiety scores in subsequent procedures.33
Comparing the results of our study with those of other authors poses challenges.
Brochard et al.26 found pain above 9 points in the CHEOPS scale in 38% of children that received injections while sedated with nitrous oxide and EMLA cream, who required a higher level of analgesia to perform the procedure. In our study, nitrous oxide was fairly effective in reducing pain in children with CP treated with BoNTA injections.
The results of this study are consistent with the findings of Gambart et al.,34 who assessed pain in children managed with nitrous oxide and an eutectic mixture of lidocaine and prilocaine (EMLA cream) and concluded that the anaesthesia protocol was inadequate in 1 out of 2 children (50%), with a greater level of pain in children receiving more than 8 injections.
Although treatment with BoNTA is effective and generally well tolerated,35 parents may worry about the pain associated with the procedure.36 In fact, the pain caused by injection has been reported as a reason for abandoning treatment with BoNTA in children.37
In our study, the level of pain differed significantly based on the selected SAP.
The proportion of parents that considered that the procedure was stressful to their children was low in Groups I and III and relatively low in the remaining 2 groups. These findings suggest that selection of the appropriate SAP significantly increases parental satisfaction with the procedure.
Although the comfort of the patient is important, it must be weighed against the risks posed to the patient. Children with CP may be at increased risk of complications of general anaesthesia,38,39 although we did not observe any adverse effects in any of the groups under study.
While the proportion of patients with a level of pain of 2 or less was significantly greater in the general anaesthesia group, this approach cannot be recommended for all patients. In addition, performance of anaesthetic procedures in patients with special needs may be challenging, while assessment of acute pain is complicated in children with significant cognitive impairments.27,34,39
The cooperation of the patient allowed performance of the intervention in most patients in all groups under study. This further corroborates the crucial importance of selecting an appropriate SAP.
We did not find significant differences in pain based on whether needle insertion was guided by ultrasound or based on anatomical landmarks. However, we ought to note that we did not originally design the study to assess the impact of insertion technique on the pain experienced by the patient. Our study had a power of 40% to detect a mean difference in pain of 1.8 points with a level of significance of 0.05.
Authors of previous studies have indicated the importance of using ultrasound to guide injection of BoNTA in different muscles.23,24,40 However, these studies were focused on the effectiveness of BoNTA rather than the pain experienced during the procedure.
Lastly, the only variable under study included in the model that was significantly associated with pain was age. This may be related to the fact that cooperative patients were assigned to Group I (topical anaesthetic), while patients that had been uncooperative in previous injections were assigned to the general anaesthesia group. Furthermore, patients in Group III were older compared to patients in Group II.
Limitations of the studyWe ought to mention certain limitations of the study that must be taken into account when interpreting its results. The first limitation is that the study was conducted in a single centre and a small sample of patients. To minimise the impact of this factors we made a sample size calculation before starting data collection. The second limitation was that it was not a blind study. The individual responsible for collecting data on pain was aware of the SAP that had been used, which may have been a source of bias.
ConclusionsThe results of our study demonstrate the importance of selecting an adequate sedation and analgesia protocol to reduce the stress associated with BoNTA injections in children with CP.
FundingWe received funding from Allergan Ltd. to support the writing of the manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cantador-Hornero M, Jiménez-Espuch P, de Torres-Garcia I, Contreras-Jiménez M, Martínez-Mezo GL, Morales de los Santos JM, et al. Protocolo sedoanalgésico para la infiltración de toxina botulínica A en parálisis cerebral. An Pediatr (Barc). 2019;91:317–327.