Patients with sickle cell disease exhibit different patterns in pulmonary function tests. In particular, there is little evidence on the fractional exhaled nitric oxide (FeNO) test, and its value ranges and its interpretation in these patients have been under debate in recent years.
MethodsWe conduced a cross-sectional, observational and descriptive study between November 2021 and January 2023 including patients aged 6–18 years with sickle cell disease able to perform the FeNO test. We applied the GLI-2012 reference values and the ERS/ATS standards. We defined statistical significance as P < 0.05.
ResultsThe sample included 43 patients with a median age of 12 years (IQR, 10−15). We did not find an association between significantly elevated FeNO (≥25 ppb) and the diagnosis of asthma (P = 0.37), an obstructive pattern in spirometry (P = 0.67), a positive bronchodilator test (P = 0.53), clinical bronchial hyperreactivity in the context of cold or flu-like symptoms (P = 0.48), cough with exercise (P = 0.42) or nocturnal cough (P = 1.0), but found an association with peripheral eosinophilia (P < 0.01).
ConclusionsWe found no association between FeNO values and the classic features of asthma (clinical or spirometric) in patients with sickle cell disease. Therefore, airway inflammation mechanisms are probably different in these patients.
Los pacientes con enfermedad de células falciformes presentan diferentes patrones en el estudio de función pulmonar. En concreto, el FeNO ha sido poco estudiado y sus valores e interpretación en estos pacientes han sido objeto de debate en los últimos años.
Material y métodosEstudio observacional, descriptivo y de corte transversal realizado entre noviembre de 2021 y enero de 2023 que incluye a pacientes de 6 a 18 años con enfermedad de células falciforme capaces de realizar el examen de FeNO. Se utilizaron valores de referencia GLI-2012 y estándares de ERS/ATS. Significación p < 0.05.
ResultadosSe incluyeron 43 pacientes, con una mediana de edad de 12 años (RIC 10−15). Se consiguieron espirometrías en 34 pacientes, mostrando 20 (59%) un patrón normal, 9 (26%) un patrón obstructivo y 5 (15%) un patrón restrictivo. El rango de valores de FeNO fue de 5−50 ppb con una mediana de 14 ppb (RIC 10–23 ppb). No se encontró relación entre un FeNO significativamente elevado (≥ 25 ppb) y el diagnóstico de asma (p 0,37), el patrón obstructivo en espirometría (p 0,67), una prueba broncodilatadora positiva (p 0,53), clínica de hiperreactividad bronquial en procesos catarrales (p 0,48), tos con el ejercicio (p 0,42) o tos nocturna (p 1,0); sí con la presencia de eosinofilia periférica (p < 0,01).
ConclusionesNo se demostró asociación entre el valor de FeNO y los indicadores clásicos de asma, tanto clínicos como espirométricos, en pacientes con enfermedad de células falciforme, por lo que es probable que estos pacientes presenten mecanismos de inflamación de la vía aérea diferentes.
Sickle cell disease (SCD) or drepanocytosis is an autosomal recessive disorder defined by the presence of haemoglobin S (Hb S) in red blood cells.1 It is characterised by intermittent vaso-occlusive events and chronic haemolytic anaemia, with multisystemic involvement during the course of disease.1,2
This disease causes respiratory complications, both acute and chronic, chief among them acute chest syndrome (ACS), pulmonary hypertension, pulmonary fibrosis and obstructive sleep apnoea/hypopnoea syndrome (OSAHS), which cause significant morbidity and mortality in patients with SCD.2–5
With regard to lung function, paediatric patients usually present obstructive changes, with the restrictive pattern predominating in adolescents and adults.6–16 This predominant obstructive pattern in the paediatric stage has classically translated into a high frequency of asthma diagnosis in patients with SCD. However, the assessment of asthma in these patients can be challenging, for, despite the seemingly higher incidence of obstruction and bronchial hyperresponsiveness (BHR), many patients do not meet the clinical criteria commonly used to define asthma in paediatrics.2,4
Thus, the use of markers commonly associated with eosinophilic airway inflammation and thus with ‘Th2 asthma’ could be useful to support or rule out the diagnosis of asthma in these patients. However, the role of fractional exhaled nitric oxide (FeNO) in patients with SCD and possible asthma remains controversial.17–23
Therefore, the main objective of our study was to assess the usefulness of FeNO in the follow-up of patients with SCD and its association with clinical, spirometry and laboratory markers of asthma/BHR in this subset of patients.
Material and methodsWe carried out an observational, descriptive and cross-sectional study in a high complexity hospital (level IIIC) and reference unit in red blood cell disorders between November 2021 and January 2023. The study included patients aged 6–18 years with SCD that were able to perform the FeNO test. Thus, we excluded patients who were not sufficiently collaborative, had acute respiratory symptoms at the time of the assessment or for whom we were unable to obtain signed informed consent.
We collected data on demographic, clinical, laboratory and lung function variables. The previous diagnosis of asthma was clinical, based on suggestive signs and symptoms (wheezing, dyspnoea or shortness of breath, coughing and chest tightness) supported, whenever possible, by compatible findings of lung function testing and the clinical and functional response to bronchodilators.24 We defined eosinophilia as an elevated eosinophil count greater than 500/μL.25
Assessment of lung functionThe methods used to assess lung function were forced spirometry (with bronchodilator response [BDR] testing) and the FeNO test.
We used a MasterScreen Combi spirometer with JLab version 5.31 (Jaeger Corp, Omaha, NE, USA), and the software package SentrySuite (Vyaire Medical, Mettawa, IL, USA) with the operating system Windows-7 professional (Microsoft, Redmond, Washington) adhering to the acceptability and reproducibility criteria standardised based on the latest American Thoracic Society and European Respiratory Society (ATS/ERS) statement.26
The spirometry parameters under study were: forced vital capacity (FVC), forced expiratory volume in one second (FEV1) and the FEV1/FVC ratio. Lung function was estimated using the standardised equations of the Global Lung Initiative (GLI) from 2012,27 recording both absolute values and z scores. We defined lung function patterns as follows: normal (FEV1/FVC z ≥ −1.64, FEV1 z ≥ −1.64 and FVC z ≥ −1.64), obstructive pattern (FEV1/FVC z < −1.64), restrictive (FVC z < −1.64) or mixed (meeting criteria for restrictive and obstructive).28
In the BDR test, salbutamol (400 μg) was delivered through an inhaler attached to a spacer. We considered the response positive if following its administration there was an increase in the FEV1 of 12% or greater compared to the value obtained in the baseline spirometry.29 We included patients with a positive BDR test at the time of the study and those with a history of a positive BDR test in the previous year.
The FeNO test was performed with the Circassia NiOX Vero device (Circassia AB, Uppsala, Sweden). The tests consists in fully emptying the lungs followed by a deep inspiration near the total lung capacity through the breathing handle of the device, which delivers air free of nitric oxide to the patient. This is followed by a slow, sustained exhalation over 10 s with a steady flow rate (50 mL/s) against the restrictive mouthpiece, after which the level of nitrogen (NO) is measured in the exhaled gas. We obtained 3 valid measurements, and the final result was the mean value for the 3 manoeuvres. This test was conducted before the spirometry. We documented the absolute values of nitric oxide measured in parts per billion (ppb). We considered FeNO values of 25 ppb or greater elevated.17,30
Statistical analysisWe summarised quantitative data as median and interquartile range (IQR) and categorical data as absolute and relative frequencies. For the bivariate analysis, we used the Mann-Whitney U test or Kruskal-Wallis test for quantitative variables and the χ2 test for qualitative To assess the association between quantitative variables, we used the Spearman correlation coefficient. Statistical significance was defined as a P value of less than 0.05. The analysis was performed with the software Statistical Package for the Social Sciences (SPSS), version 26 (Minnesota, USA).
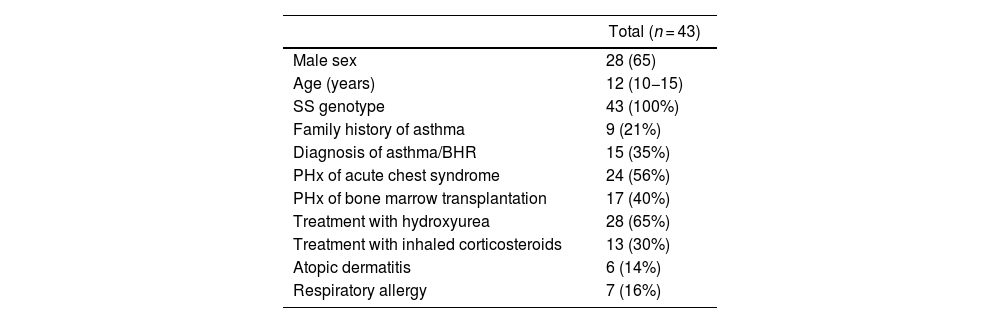
ResultsThe sample included 43 patients with a median age of 12 years (IQR, 10−15), of who 28 (65.1%) were male. Table 1 describes the characteristics of the sample.
Characteristics of the sample.
| Total (n = 43) | |
|---|---|
| Male sex | 28 (65) |
| Age (years) | 12 (10−15) |
| SS genotype | 43 (100%) |
| Family history of asthma | 9 (21%) |
| Diagnosis of asthma/BHR | 15 (35%) |
| PHx of acute chest syndrome | 24 (56%) |
| PHx of bone marrow transplantation | 17 (40%) |
| Treatment with hydroxyurea | 28 (65%) |
| Treatment with inhaled corticosteroids | 13 (30%) |
| Atopic dermatitis | 6 (14%) |
| Respiratory allergy | 7 (16%) |
BHR, bronchial hyperresponsiveness; PHx, personal history.
Qualitative data expressed as absolute frequency and percentage. Quantitative data expressed as median and interquartile range.
Spirometry measurements that met the quality standards were achieved in 34 patients. The median FEV1 z score was −1.31 (IQR, −2.01 to −0.67) and the median FVC −1.10 (IQR, −1.74 to −0.78). The median FEV1/FVC ratio was 85.23% (IQR, 81.17–89.92) and the median FEV1/FVC z score −0.41 (IQR, −0.72 to 0.30). The observed pattern was normal in 20 patients (20/34; 59%), obstructive in 9 (9/34; 26%) and restrictive in 5 (5/34; 15%). Eight patients (8/34; 24%) had a positive BDR at the time of the assessment or a history of a positive result within the previous year. When we compared patients with and without a history of bone marrow transplantation, we did not find significant differences in the FEV1 (P = 0.648), the FVC (P = 0.382) or the FEV1/FVC (P = 1.000). At the time of the assessment, none of the patients who had undergone haematopoietic stem cell transplantation had a diagnosis or suspected diagnosis of graft versus host disease involving the lungs.
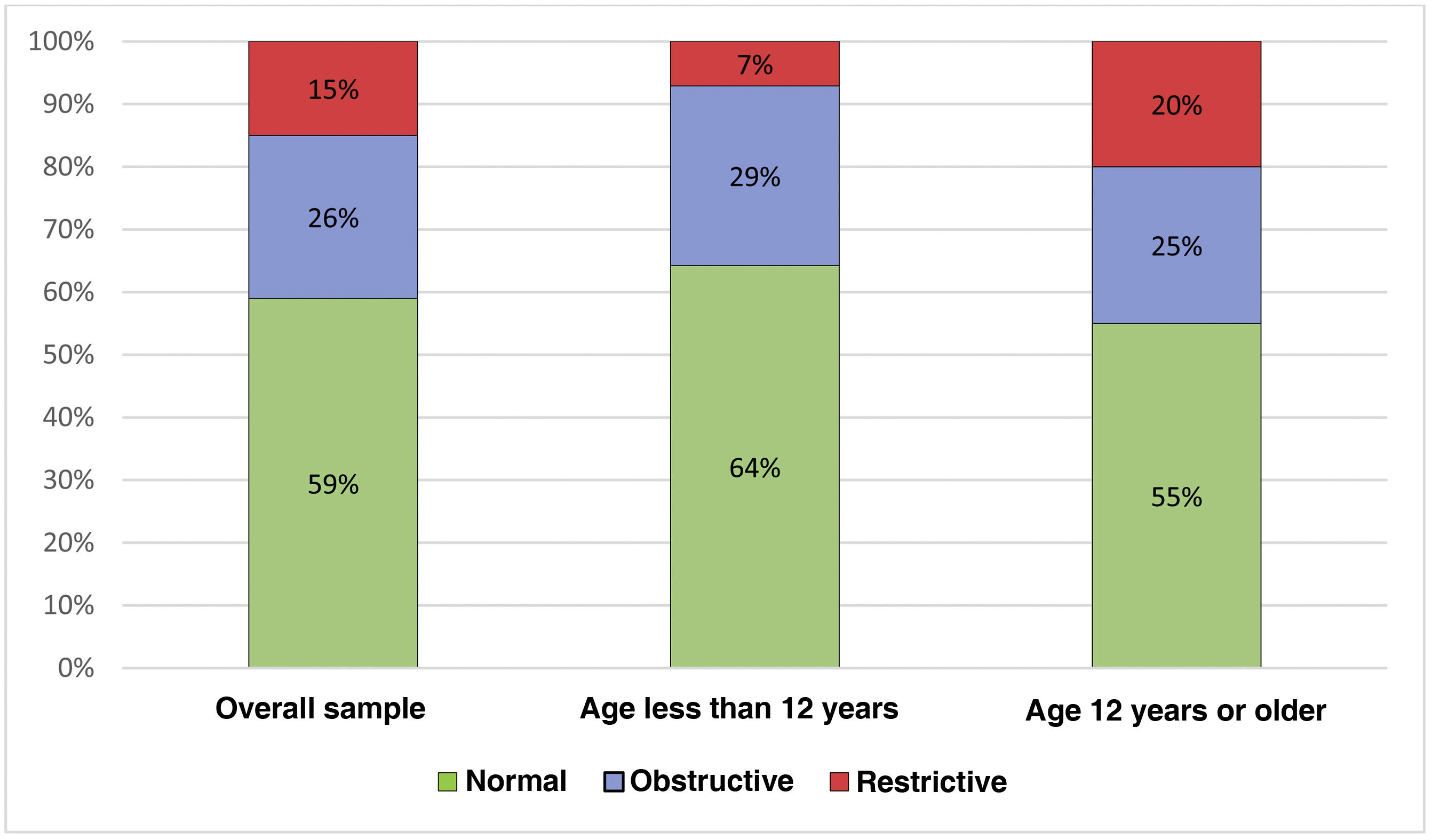
When we divided the sample by age, in the group under 12 years, 9/14 (64.28%) of patients exhibited a normal pattern, 4/14 (28.6%) an obstructive pattern and 1/14 (7.11%) a restrictive pattern, while in the group over 12 years, 11/20 (55.0%) exhibited a normal pattern, 5/20 (25%) an obstructive pattern and 4/20 (20%) a restrictive pattern, with no significant differences between the groups (P = 0.182) (Fig. 1). When it came to the FVC z score, the median value in the group aged less than 12 years was −0.85 (IQR, −0.20 to −1.19) compared to −1.55 in the group aged more than 12 years (IQR, −0.84 to −2.20), with significant differences between the two groups (P = 0.012).
In the FeNO test, the values ranged between 5 and 50 ppb with a median of 14 ppb (IQR, 10−23 ppb).
We found significantly elevated FeNO values (≥25 ppb) in 9 patients (9/43; 21%).
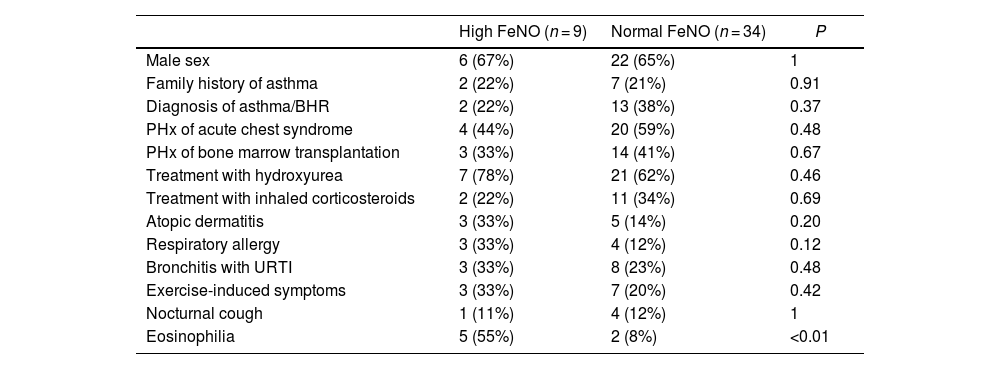
There was no association between high FeNO values (≥25 ppb) and the diagnosis of asthma/BHR or any of its classical symptoms separately. We did find an association between high FeNO values and the presence of peripheral eosinophilia (Table 2).
Comparison of patients with high vs normal FeNO values.
| High FeNO (n = 9) | Normal FeNO (n = 34) | P | |
|---|---|---|---|
| Male sex | 6 (67%) | 22 (65%) | 1 |
| Family history of asthma | 2 (22%) | 7 (21%) | 0.91 |
| Diagnosis of asthma/BHR | 2 (22%) | 13 (38%) | 0.37 |
| PHx of acute chest syndrome | 4 (44%) | 20 (59%) | 0.48 |
| PHx of bone marrow transplantation | 3 (33%) | 14 (41%) | 0.67 |
| Treatment with hydroxyurea | 7 (78%) | 21 (62%) | 0.46 |
| Treatment with inhaled corticosteroids | 2 (22%) | 11 (34%) | 0.69 |
| Atopic dermatitis | 3 (33%) | 5 (14%) | 0.20 |
| Respiratory allergy | 3 (33%) | 4 (12%) | 0.12 |
| Bronchitis with URTI | 3 (33%) | 8 (23%) | 0.48 |
| Exercise-induced symptoms | 3 (33%) | 7 (20%) | 0.42 |
| Nocturnal cough | 1 (11%) | 4 (12%) | 1 |
| Eosinophilia | 5 (55%) | 2 (8%) | <0.01 |
FeNO: fractional exhaled nitric oxide; PHx, personal history; URTI, upper respiratory tract infection.
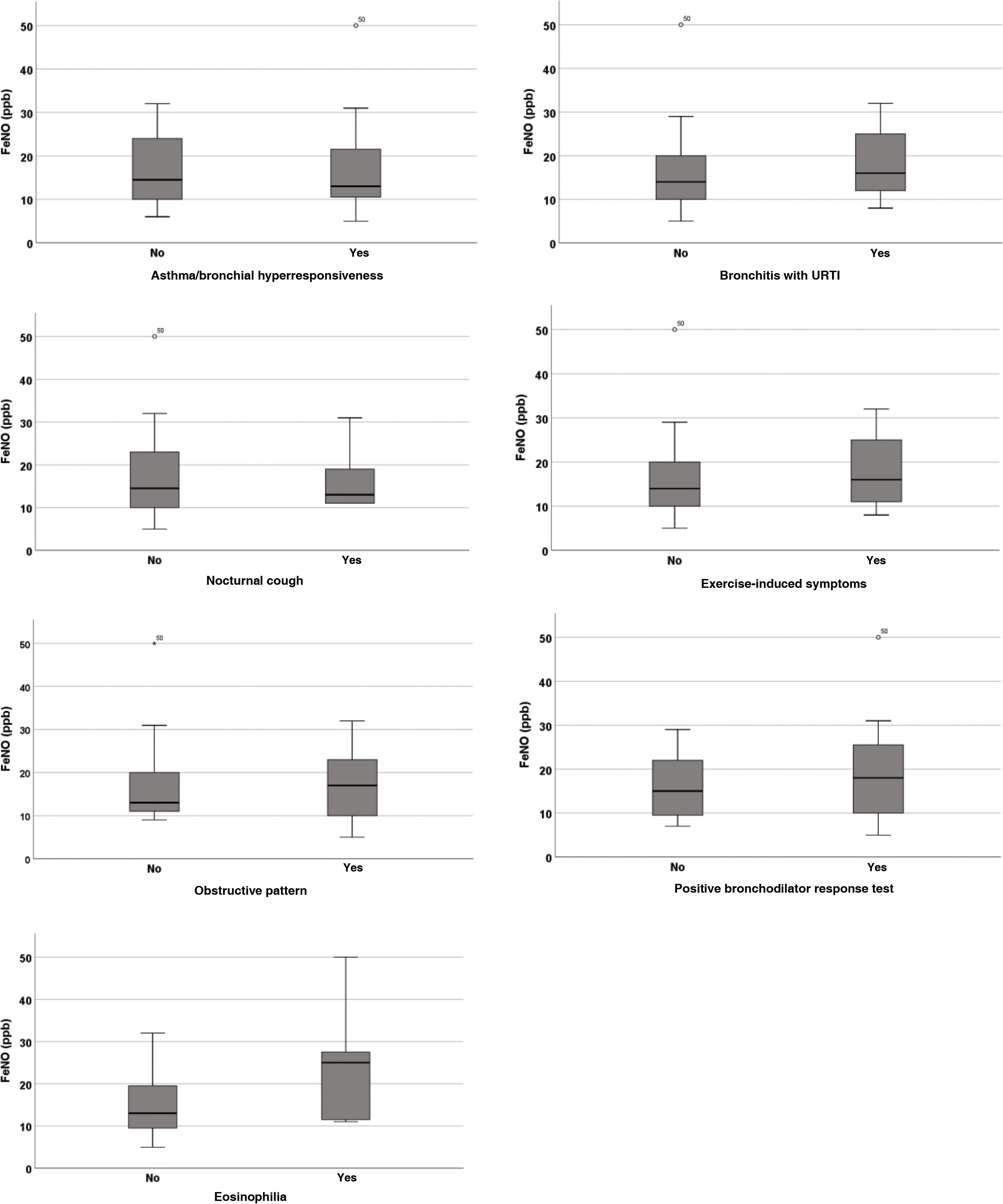
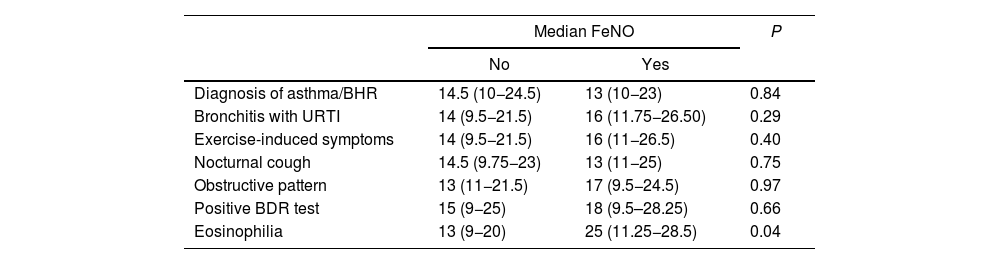
We found similar results when we evaluated these same variables with the raw FeNO values (Table 3, Fig. 2).
Comparison of FeNO values with categorical variables.
| Median FeNO | P | ||
|---|---|---|---|
| No | Yes | ||
| Diagnosis of asthma/BHR | 14.5 (10−24.5) | 13 (10−23) | 0.84 |
| Bronchitis with URTI | 14 (9.5−21.5) | 16 (11.75−26.50) | 0.29 |
| Exercise-induced symptoms | 14 (9.5−21.5) | 16 (11−26.5) | 0.40 |
| Nocturnal cough | 14.5 (9.75−23) | 13 (11−25) | 0.75 |
| Obstructive pattern | 13 (11−21.5) | 17 (9.5−24.5) | 0.97 |
| Positive BDR test | 15 (9−25) | 18 (9.5–28.25) | 0.66 |
| Eosinophilia | 13 (9−20) | 25 (11.25−28.5) | 0.04 |
BDR, bronchodilator response; BHR, bronchial hyperresponsiveness; FeNO, fractional exhaled nitric oxide; URTI, upper respiratory tract infection.
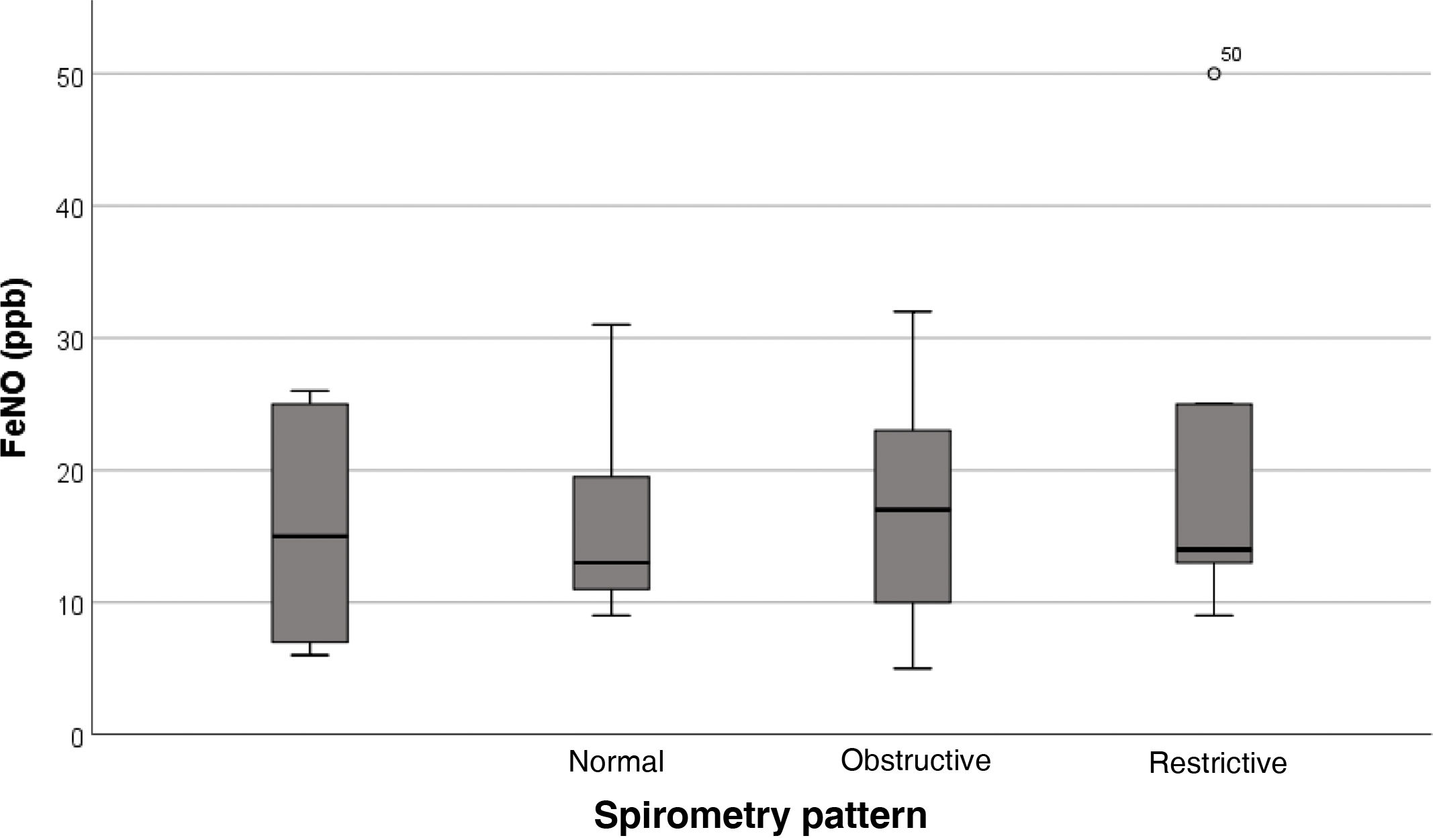
Regarding the association of FeNO with lung function, we found no significant differences in raw FeNO values based on the spirometry pattern (P = 0.829) (Fig. 3).
Focusing on the obstructive pattern, we found that it was present in 33% (2/6) of patients with elevated FeNO values compared to 25% (7/28) of patients with normal FeNO values, with no significant differences between these groups (P = 0.672).
Similarly, there were no differences in the proportion of patients with a positive BDR between the group with high FeNO values (2/6; 33%) and the group with normal FeNO values (6/28; 21%) (P = 0.527). We found no differences in the FeNO values of patients with BDR at the time of the assessment versus those with a previous positive result (P = 0.724).
There was also no association between the FeNO value and the FEV1 z score (r = −0.2; P = 0.121).
DiscussionAs we previously noted, lung function in patients with SCD is a controversial subject in the literature.6–16 In terms of spirometry findings, while some studies have reported the restrictive pattern as the one most frequently found in children and adolescents,7–16 most recently published works, including this one, have found a predominance of the obstructive pattern as the most common abnormality in the paediatric age group.6–12 Thus, in our study, the frequency of the obstructive pattern (26%) was similar to that reported in other studies, like those by Koumbourlis et al.13 (22%) or the second cohort studied by Lunt et al.6 (24%), and slightly lower than the one reported by Lunt et al.6 for their first cohort (34%) and by Angel et al.11 (31%). The literature shows that the obstructive pattern is discernible as early as infancy.7 The pathophysiological mechanism, while not clearly elucidated, appears to be associated with systemic inflammation, markers of haemolysis and the calibre of pulmonary vessels.6,12,31
Longitudinal studies like those by Lunt et al.6 or MacLean et al.14 describe an increasing prevalence of the restrictive pattern with age. These and other studies included in the meta-analysis published by Taksande et al.12 show that chronic pulmonary inflammation and recurrent episodes of ACS contribute to fibrotic changes in the lung parenchyma which in turn promote the described progression to restrictive pulmonary disease, which is also observed in older adults.31–33 In our study, we found a significant decrease in FVC in older patients and, although the difference was not statistically significant, an increase in the prevalence of the restrictive pattern in this age group.
As for the FeNO, its significance in SCD is, as discussed above, difficult to interpret. In our study, the median FeNO was 14 ppb, which was similar to previous studies. In fact, several studies have found even lower FeNO values in patients with SCD compared to healthy individuals.21,22 A recent review described how haemolysis consumes nitric oxide through different pathways in patients with SCD, explaining the observed decrease in the FeNO compared to healthy individuals, and resulting in vasoconstriction and inflammation.23 In contrast, Radhakrishnan et al.18 found an increase in the FeNO in the airway of patients with SCD compared to healthy subjects.
A possible explanation for the heterogeneity of the findings in the literature could be the source of the exhaled nitric oxide. Thus, studying the FeNO in multiple exhalation flows, Lunt et al.20 found low NO concentrations in the airway and elevated concentrations only at the alveolar level, which was associated with an increased blood flow, suggesting that the hyperdynamic pulmonary circulation characteristic of patients with this chronic anaemia could explain the FeNO elevation.
Although multiple studies have analysed asthma and BHR in patients with SCD, to date, only Cohen et al.17 have directly evaluated the association with FeNO values. In our study, in agreement to the findings of these authors, we found an association between an elevated FeNO (>25 ppb) and the presence of peripheral eosinophilia in these patients. However, like Cohen et al.,17 we did not find an association between elevated FeNO values and typical markers of atopy, such as dermatitis or allergies, nor between elevated FeNO values and the diagnosis of asthma, the obstructive pattern on spirometry, a positive BDR or the presence of classical BHR symptoms (wheezing, activity intolerance, nocturnal cough). These findings were also corroborated by Lunt et al.,20 who also did not find an association between NO levels in the airway and the obstruction evinced in lung function testing. Chaudry et al19 found that while methacholine sensitivity is associated with eosinophilia and FeNO elevation in healthy subjects, this is not the case in patients with sickle cell disease, which suggests a different pathophysiology.
In the same line, an exhaustive review by De et al.23 demonstrated that in patients with SCD, airway obstruction and BHR are associated with immunoglobulin E (IgE) levels and markers of haemolysis such as lactate dehydrogenase (LDH), but not with the eosinophil count or sensitization to respiratory allergens as evinced by allergy skin tests or elevation of exhaled NO, which are classic markers of atopy and eosinophilic inflammation of the airway. These findings, except in the case of the eosinophil count, are consistent with those of our study and suggest that while IgE could play a role in airway inflammation in patients with SCD, the underlying mechanism could differ from that of airway inflammation in allergic asthma, which, in addition to IgE, involves other mediators such as eosinophils, sensitization to allergens and, ultimately, inflammation mediated by Th2 cells.
In this regard, De et al.23 described how the abnormal haemoglobin of SCD causes changes in the shape and surface properties of red blood cells that promote endothelial inflammation, adhesion of neutrophiles, monocytes and platelets, the release of proinflammatory cytokines and the activation of the coagulation cascade. All of this, combined with the aforementioned decrease in the concentration of NO and other mechanisms, gives rise to a proinflammatory state. Furthermore, other inflammatory pathways, such as leukotriene activation, have been demonstrated in both humans and mice with SCD.34–36
In short, airway obstruction and BHR in some patients with SCD seems to differ from the classic Th2 asthma phenotype, which involves systemic inflammation, lung injury caused by vaso-occlusive episodes and the intravascular haemolysis associated with SCD. In this regard, De et al.23 propose the use of a term other than “asthma” to refer to bronchial obstruction problems in this subset of patients: “sickle cell-related airway inflammation.” Beyond the theoretical relevance of such disquisitions, it may be that the management of these patients is approached from too narrow a perspective. Another aspect contributing to this issue is the lack of therapeutic options, which at times results in the oversimplification of treatment in patients with SCD, so that bronchial obstruction is managed as if it were Th2 asthma. Therefore, further research is required to delve deeper in the pathophysiology and treatment response of patients with SCD, studying drugs that target different inflammatory pathways including those involving neutrophils, basophiles and leukotrienes, among others.
There are limitations to the study. First of all, it was conducted in a single centre, although, since it was the reference centre for SCD, it covered a large geographical area. On the other hand, FeNO was only measured in a single exhalation flow which, as we noted above, may give rise to heterogeneous results. When it comes to the cut-off FeNO value, it was set at 25 ppb based in the only similar study found in the literature17 and the most recent recommendations for diagnosis of asthma in the paediatric population of the ERS,29 although this threshold is under debate and has not been validated in these patients. Although we took into account treatment with inhaled corticosteroids, we did not assess adherence to it, which could affect FeNO values. Another potential limitation is that we accepted previous positive BDR results as valid in the study. The reason is that this test offers a limited sensitivity and that the study of FeNO values in patients with confirmed BHR was particularly relevant. At any rate, we compared the patients with a positive BDR result in the study period with those with a recent positive result to assess the potential risk of bias, and we found no differences in the FeNO values between the two groups. Last of all, the cross-sectional design of the study precluded performance of a prospective analysis of the risk of ACS in relation to FeNO values, a subject that is currently attracting interest.
There are also strengths to the study. It is the first paediatric study assessing lung function in patients with SCD in Spain. It is also one of the few studies in the global literature that directly assess the association between FeNO and asthma/BHR in this specific subset of patients.
In conclusion, our study, like those in the reviewed recent literature, found that the normal pattern was most common in paediatric patients with SCD, while the obstructive pattern was the most frequent abnormal pattern on spirometry, although there was an increase in the frequency of the restrictive pattern in older patients. Furthermore, we found no association between FeNO values and the classical indicators of asthma, both clinical and on spirometry, so it is likely that these patients have different mechanisms of airway inflammation. Further studies are needed to investigate the nature of inflammation in patients with SCD and its targeted treatment.
Ethical considerationsThe study was approved by the ethics committee of the hospital. We obtained informed consent for all the included patients.
FundingThis research did not receive any external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.