Coeliac disease is a common condition for which the only current treatment is a gluten-free diet. Adherence to this diet is not always easy and is associated with a reduction in quality of life for the patient and their family. Non-adherence is associated with complications of varying severity. The lack of control at the outpatient care level in a high percentage of these patients evinces the need to improve follow-up protocols and the approach to care delivery with coordination of paediatric gastroenterology units (PGU) and primary care paediatricians. With this aim in mind, the present document was developed by consensus to offer a set of recommendations adapted to our region, based on the recent recommendations published by the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), and with participation of the pertinent scientific societies, including those concerning the adult population, for the management and follow-up of adolescents and the transition to adult care.
La enfermedad celíaca es una patología frecuente y cuyo único tratamiento en el momento actual es la dieta sin gluten. El seguimiento de esta dieta no siempre es fácil e implica limitaciones en la calidad de vida del paciente y su familia. La no adherencia se asociaría a complicaciones de distinta gravedad. La falta de control de estos pacientes en consultas en un alto porcentaje de casos plantea la necesidad de mejorar los protocolos de seguimiento y de abordarlos de forma coordinada entre las Unidades de Gastroenterología Pediátrica (UGP) y los pediatras de Atención Primaria. Con ese objetivo se han consensuado en este documento, basándose en las recomendaciones recientemente publicadas por la Sociedad Europea de Gastroenterología, Hepatología y Nutrición Pediátrica (ESPGHAN), un conjunto de recomendaciones adaptadas a nuestro entorno y contando con la participación de las Sociedades Científicas implicadas, incluyendo a las Sociedades de adultos para el abordaje del seguimiento del adolescente y de la transición de cuidados a los profesionales de adultos.
Coeliac disease (CD) is an immune-mediated systemic disorder elicited by gluten in genetically susceptible individuals and characterised by the presence of a variable combination of clinical manifestations, CD-specific antibodies, HLA-DQ2 or HLA-DQ8 haplotypes, and enteropathy.1
The primary care (PC) paediatricians is usually the provider that suspects and makes the initial assessment of the disease, and the diagnosis is confirmed by the paediatric gastroenterologist.2 The only available treatment is the gluten-free diet,3 which achieves resolution of symptoms and recovery of the intestinal mucosa. Although adherence to the diet has an impact on the quality of life of the patient and the family, lack of adherence carries a risk of complications of varying severity.
There is evidence that inconsistent or absent follow-up is associated with poor adherence to the GFD, so high-quality clinical monitoring is required through the lifespan. Self-management without medical guidance or follow-up visits is not recommended.4
In spite of this, up to 35% of affected patients do not attend scheduled follow-up visits,5,6 even with the implementation of proactive follow-up protocols in paediatric gastroenterology units (PGUs). In order to improve this, we propose implementation of follow-up in coordination with PC.
The aim of this document is to offer a consensus-based set of recommendations based on the follow-up guidelines recently published by the European Society of Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN4 adapted to the circumstances and diversity of the Spanish population.
MethodsWorking group and general structure of the documentThe working group was composed of 29 experts who represented the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (SEGHNP, Spanish Society of Paediatric Gastroenterology, Hepatology and Nutrition), Asociación Española de Pediatría de Atención Primaria (AEPap, Spanish Association of Primary Care Paediatrics), Sociedad Española de Pediatría Extrahospitalaria y Atención Primaria (SEPEAP, Spanish Association of Ambulatory and Primary Care Paediatrics), Sociedad Española de Enfermedad Celíaca (SEEC, Spanish Coeliac Disease Society), Asociación Española de Gastroenterología (AEG, Spanish Association of Gastroenterology), Sociedad Española de Patología Digestiva (SEPD, Spanish Association of Digestive Diseases), Sociedad Española de Medicina de Familia y Comunitaria (SEMFYC, Spanish Society of Family and Community Medicine), Sociedad Española de Médicos Generales y de Familia (SEMG, Spanish Society of General and Family Physicians) and Sociedad Española de Médicos de Atención Primaria (SEMERGEN, Spanish Society of Primary Care Physicians). The group also had the collaboration of patient associations: Federación de Asociaciones de Celíacos de España (FACE, Federation of Coeliac Disease Associations in Spain), Asociación Celíacos de Cataluña (Catalan Association of Coeliac Disease) and Asociación de Celíacos y Sensibles al Gluten de Madrid. (Madrid Association of Coeliac Disease and Gluten Sensitivity)
Fifteen clinical questions were chosen by consensus, structured into 3 sections: immediate follow-up after diagnosis (active CD), long-term follow-up (CD in remission) and follow-up of adolescents (14–18 years, including the transition to adult care). The questions were then divided among the working group members for their study and review based on their area of expertise and care setting/level.
Literature reviewThe literature review started off with the previous review conducted by the ESPGHAN with sources published through March 2020.4 It was then completed with the most relevant sources published through October 2023 on the subjects of “coeliac AND follow-up” and “coeliac AND transition” (PubMed/Medline) and position statements of scientific societies associated with the subject.
Development of the documentThe reviews focused on each of the questions were summarised in a series of statements and recommendations that were subsequently debated by the whole group to reach a consensus. Each recommendation was subjected to an anonymous vote, with voters choosing among the following options: agreement (A); abstention (Abs); disagreement (D). Consensus for a recommendation was defined as agreement by 85% of participants.
ResultsThe search of the literature published after the ESPGHAN 2022 recommendations yielded 196 publications on the follow-up of CD and 59 on care transition, out of which 57 and 21, respectively, were selected for closer reading, with the 40 most relevant included in the review.
Section 1. Immediate follow-up after diagnosis (active CD)Question 1. Which providers should be involved in the follow-up?The initial management should be conducted by a paediatric gastroenterologist, the professional best qualified for management of CD.4 Whenever possible, guidance by a dietician/nutritionist is also recommended.
Question 2. How should follow-up be organised?Frequency of visitsGiven the lack of evidence on the subject, the current recommendation is to schedule a first follow-up visit 3–6 months after initiation of the GFD, or earlier if the clinical condition of the patient so requires. Subsequent visits will be scheduled every 6–12 months depending on the course of disease.4
Follow-up assessment methodsClinical evaluation:
Follow-up visits should include an assessment of the signs and symptoms that were present at diagnosis to verify their resolution.4 The development of new symptoms may signal the development of comorbidities associated with CD.
It is important to monitor nutritional status and growth, especially in patients with impaired growth at the time of CD diagnosis. Catch-up growth is expected within 6 months of initiation of the GFD. Other causes of short stature should be ruled out if catch-up linear growth does not occur after 1 year of strict adherence to the diet, especially in prepubertal patients.
Diagnostic tests:
- -
IgA tissue transglutaminase (tTG) antibody testing is the preferred choice for serological monitoring. In half of paediatric cases, levels become negative at 1 year of the GFD,4,7 usually with greater delay in patients with higher antibody titres at diagnosis and when testing is done with chemiluminescence techniques.7 Follow-up serology testing to verify normalization of antibodies is recommended starting from 1 year of the GFD, with re-evaluation of patients in whom positive serology persists longer than 2 years.
- -
At the time of diagnosis, the patient may have decreased levels of micronutrients such as iron, folate, vitamin B12 or vitamin D, which should be monitored until they normalise, with consideration of supplementation in the case of anaemia or significant deficiency.4 Thus, measurement of micronutrient levels will be performed throughout the follow-up in select cases.8,9
- -
There may be mild elevation of liver enzymes at diagnosis, especially in younger patients, which usually resolves by 1 year of the GFD.10,11 Autoimmune liver disease should be considered in the differential diagnosis of persistent hypertransaminasaemia.12
- -
Coeliac disease is associated with an increased risk of autoimmune thyroid disease.4 Although there is no evidence on the need to monitor thyroid function during follow-up, regular measurement of thyroid-stimulating hormone (TSH) seems reasonable in patients with other autoimmune diseases, especially type 1 diabetes (T1D), during puberty (especially in female patients) and in the case of persistent positive serology for CD.13
- -
Decreased bone mineral density (BMD) has been observed in paediatric patients with CD at the time of diagnosis,14 with subsequent improvement with the GFD. However, studies assessing the risk of bone fracture in paediatric patients with CD have yielded contradictory results, so the current evidence does not support the recommendation of a bone density scan at diagnosis or during the follow-up.15 A bone density scan should be performed in patients with risk factors such as suspected bone disease (more than 2–3 long bone fractures or vertebral fracture in absence of local disease or high-energy trauma), persistent malabsorption syndrome, potential CD with a regular diet or lack of adherence to the GFD.
The usefulness of clinical evaluations and symptom monitoring to assess adherence to the GFD is limited, as there is an increasing number of oligosymptomatic patients. Standardised dietary interviews conducted by a dietitian/nutritionist or dietary questionnaires have proven more sensitive for the identification of dietary transgressions.4 There are brief and easy to administer questionnaires16 that may be useful in clinical practice, but they have not been validated in paediatric patients, with the exception of the Coeliac Dietary Adherence Test (CDAT), validated for use in patients aged more than 12 years.17
Laboratory testsNormalization of tTG antibody titres can serve as an indirect marker of mucosal healing. Any elevation thereafter can be indicative of significant dietary transgressions, but negative results do not guarantee strict adherence to the diet.4
Role of gluten immunogenic peptidesFollowing gluten consumption, gluten immunogenic peptides (GIPs) are detectable in urine for 3–24 hours and in faeces for up to 7 days, and the test offers a good sensitivity and specificity. Repeated measurement of GIPs improves performance of this test for monitoring of adherence in the long term, as opposed to isolated transgressions. The interpretation of results has yet to be standardised, so it is recommended that they are used in combination with the other methods described above.4,18–20
Question 4. Frequent and/or specific problems in follow-upPersistence of symptomsAlthough symptoms tend to resolve within one year, more than half of the patients may experience some symptoms after that point in spite of the GFD.21 Dietary transgressions are the most frequent cause, but symptoms may also be due to coexisting conditions, such as functional gastrointestinal disorders.2,22
Need for biopsyHealing of the intestinal mucosa and normalization of tTG antibodies tend to occur after 2 years of the GFD. Performance of an intestinal biopsy (IB) should be considered if serology continues to be positive after 2 years of GFD with adequate adherence or if there is uncertainty regarding the original diagnosis.2,4
Refractory coeliac diseaseIn the case of suspected refractory CD (persistence of malabsorption with villous atrophy), rule out gluten intake and coexisting gastrointestinal disorders, as refractory CD is rare in the paediatric age group.4
Question 5. How should quality of life be assessed?In children with CD, quality of life (QoL) may be impaired by having a chronic disease requiring a life-long diet.4 The correct assessment of QoL requires the use of instruments specifically designed for children with CD. The two questionnaires currently available (Coeliac Disease Dutch questionnaire [CDDUX] and Celiac Disease Quality of Life Measure [CDPQOL]) have undergone translation and transcultural adaptation and validated for use in Spain23 (Appendix A, 1 and 2). In clinical practice, we recommend investigating the responses for each item to make a more thorough assessment of those with poor ratings (sad faces in CDDUX and score greater than 2 or of ‘almost never’ in the CDPQOL).
Question 6. Follow-up in special situationsUncertain diagnosisIf gluten was eliminated before the diagnosis was confirmed or after an inadequate diagnosis (criteria not met),2 a gluten challenge (GC) should be performed to reach a certain diagnosis. Human leukocyte antigen (HLA) testing can help identify patients at risk of CD. In patients with genetic risk, the first clinical and laboratory evaluation follow-up evaluation should be scheduled 1–3 months after initiation of the GC to minimise exposure to gluten, with successive follow-up evaluations every 3−6 months through 1 year. The GC should follow an established protocol, preferably avoiding critical periods of growth and development (age <5 years or puberty), with a limited gluten intake in the first year of 10−15 g (1 slice of bread has 3−5 g) and an unrestricted diet thereafter. If at 2 years of gluten exposure tTG levels continue to be negative and the patient remains asymptomatic, the probability of developing CD is low,4 although the patient should remain in follow-up and possibly undergo an IB, as there have been reports of late diagnosis.
Coeliac disease and type 1 diabetesPatients with T1D and CD tend to be asymptomatic and exhibit poorer adherence to the GFD. Notwithstanding, the recommended follow-up is the same as the one for patients with CD without T1D, with emphasis on adherence to the GFD24 and screening for thyroid disease.4
IgA deficiencyThere is no evidence supporting changes in follow-up compared to immunocompetent patients, except for testing for IgG antibodies and repeating IgA antibody testing after 4 years to rule out transient IgA deficiency. The longitudinal trend in IgG antibody levels is different compared to the trends in IgA antibodies observed in immunocompetent patients, as IgG antibodies become negative in fewer than half of patients at 2 years of diagnosis and may remain positive or fluctuate for years even if mucosal healing has been achieved.25 Repetition of the IB has been proposed in patients with persistence of positive IgG antibodies for more than 2 or 3 years, especially if there is concern about adherence to treatment.26
Potential coeliac diseaseIt is defined as the presence of tTG antibodies and a compatible HLA with normal duodenal architecture (Marsh 0–1), with or without symptoms.4 Correct diagnosis requires ensuring an adequate gluten intake and number and orientation of intestinal biopsies.27 In the case of asymptomatic potential CD, the possibility of maintaining gluten in the diet while monitoring clinical and laboratory parameters every 6–12 months will be discussed with the family/patient, as more than 50% of these patients never develop the disease,4 with close monitoring of growth and bone health as well. Repetition of the IB is recommended if symptoms develop, there is an increase in antibody titres or antibody titres continue to be positive at 2 years of follow-up. Initiation of the GFD should be considered in patients with symptomatic potential CD.4,28
Question 7. Are changes to the vaccination schedule required?Patients with CD who do not strictly adhere to the GFD or whose tTG antibody levels have not become negative are in a state of “functional hyposplenism” that makes them susceptible to infection by encapsulated bacteria (pneumococci and meningococci). When disease control is adequate, the long-term vaccine response in these patients is similar to that in the general population, and the same recommendations for vaccination apply, including for vaccination against the seasonal flu29 (Appendix A 3: vaccination table).
When it comes to the hepatitis B virus (HBV), patients with CD exhibit a diminished antibody response to the vaccine, which may be related to the HLA-DQ2 haplotype,30 but not an increased incidence of HBV infection,31 which suggests that long-lasting protection is maintained through cellular immunity.32 In consequence, the Advisory Committee on Vaccines of the AEP does not establish different recommendations for individuals with CD compared to the general population. In patients who require repeat vaccination due to vulnerability to HBV infection, the Committee recommends verification of adequate adherence to the GFD prior to vaccination.29
The PC paediatrician will be responsible for determining any necessary modifications to the vaccination schedule.
What has been discussed in this section is the basis for the recommendations summarised in Table 1.
Recommendations (active CD).
| Question 1. Which providers should be involved in the follow-up? |
| - Initial management: paediatric gastroenterologist and, whenever possible, guidance by a dietitian/nutritionist |
| Agreement: 100% |
| Question 2. How should follow-up be organised? |
| First visit: at 3−6 months from diagnosis of CD. Subsequent visits: every 6−12 months |
| Monitor: nutritional status (weight, height, growth velocity), pubertal development, changes in signs and symptoms |
| Measure tTG antibodies, preferably with the same technique used at diagnosis |
| Monitor micronutrient levels (iron, folic acid, vitamins D and B12) and liver enzyme levels if liver function tests were abnormal at diagnosis. In patients with iron deficiency anaemia, consider oral iron supplementation, especially during critical growth periods; iron supplementation is not necessary if iron levels are low in absence of anaemia. |
| Consider assessment of thyroid function, especially in patients with risk factors: T1D, puberty in girls, and persistent positive serology. |
| Consider bone density scan in patients with risk factors: suspected bone disease, poor adherence to GFD or potential CD in patients following a regular diet. |
| Agreement: 100% |
| Question 3. How can transgressions in the gluten-free diet be detected?: |
| - Assessment: symptoms, dietary interview, brief adherence questionnaires and laboratory tests. Consider measurement of GIPs to detect recent dietary transgressions. |
| Agreement: 100% |
| Question 4. Frequent and/or specific problems in follow-up |
| - Persistent symptoms under GFD: rule out dietary transgressions and assess for other causes |
| - Consider intestinal biopsy if tTG antibody titres remain positive after more than 2 years of GFD with adequate adherence or if there is uncertainty regarding the CD diagnosis |
| Agreement: 93%. Abstention: 7% |
| Question 5. Assessment of quality of life |
| Use instruments specifically developed for assessment of QoL in children with CD (CDDUX or CDPQOL) |
| Agreement: 96%. Abstention: 4% |
| Question 6. Follow-up in special situations |
| - Consider performance of gluten challenge according to a standardised protocol and under medical supervision if there is diagnostic uncertainty or diagnostic criteria were not fully met. |
| - CD associated with T1D: carry out the follow-up recommended in patients with CD, with emphasis on the evaluation for autoimmune thyroid disease |
| - IgA deficiency: serological testing with IgG antibodies |
| - Asymptomatic potential CD: if the patient follows a regular diet (with gluten), perform follow-up clinical and laboratory assessments every 6−12 months. Repeat IB if the patient develops compatible symptoms or there is an increase or persistent elevation of tTG titres |
| - Symptomatic potential CD: consider elimination of gluten from the diet with the corresponding follow-up |
| Agreement: 100% |
| Question 7. Are changes to the vaccination schedule required? |
| - In patients with adequate adherence to the GFD, the recommendations for vaccination are the same as in the general population, including the influenza vaccine. In patients with poor adherence to the GFD, it is important to ensure correct vaccination against pneumococcal and meningococcal disease. Routine assessment of the response to the HBV vaccine is not recommended, except in patients with risk factors for HBV infection |
| - The PC paediatrician will be responsible for making the necessary modifications to the vaccination schedule |
| Agreement: 89%. Abstention: 7%. Disagreement: 4% |
The follow-up of the patient with CD in remission should be multidisciplinary: paediatric gastroenterologist, PC paediatrician, nutritionist and/or specialised nurse.
Due to a lack of data on which provided would be most effective in improving adherence to the GFD, most expert groups conclude that the follow-up should be conducted by health care professionals with previous experience on the disease and the resources necessary for its management.4,33,34
Question 9. When should follow-up at PC level be considered?When the paediatric gastroenterologist considers that the patient has achieved adequate disease control, based on the following criteria:
- •
Adequate adherence to GFD.
- •
Resolution of symptoms present at diagnosis of CD.
- •
Sustained normalization of serology results in annual check-ups in at least 2 assessments.
- •
Normal growth and development.
- •
Absence of nutritional deficiencies.
Table 2 presents the criteria for follow-up at the PC level.
Criteria for referral to and from primary care/hospital.
| Referral to primary care | Referral to hospital-based care |
|---|---|
| -Disease control, absence of special situations or risk- Report by paediatric gastroenterologist including information on onset of disease, serology and/or endoscopy results, assessment of disease in family, adherence to GFD, vaccine status, comorbidities and current clinical and serological status- Knowledge of current recommendations for CD follow-up and GFD by PC team (paediatrician, nurse)- Access to paediatric gastroenterology unit- Decision made in agreement with patient and family | - Problems with diet: non-adherence for psychosocial reasons, lack of adherence to GFD or nutritionally inadequate diet, need of guidance by nutrition team and/or dietitian- Development of comorbidities: T1D, thyroid disease or autoimmune hepatitis - Recurrence of initial symptoms, development of new symptoms or delay in growth and/or development- Positive serology without clear evidence of dietary transgression |
There are patients with CD in special situations that require follow-up by a paediatric gastroenterology, even if the disease is considered to be under control, as is the case of patients with potential CD,5 T1D, IgA deficiency or other comorbidities (autoimmune hepatitis, thyroid disease, etc).
Question 10. How should it be organised?Frequency of visitsStarting from 1 year after initiation of the GFD, in patients with adequate control of the disease, follow-up visits can be scheduled every 1–2 years.
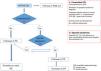
In certain situations (Table 2), referral to hospital-based care will be considered, and, likewise, if the patient is in follow-up at hospital, transfer to PC can be contemplated if the required conditions are met (Fig. 1).
Approach to follow-upThe recommendations are the same as for active CD and can be found in Table 3.
Recommendations for follow-up of patients with CD in remission.
| Question 8. Which providers should be involved? |
| - Multidisciplinary follow-up: paediatric gastroenterologist, PC paediatrician, nutritionist and/or specialised nurse |
| Agreement: 94%. Abstention: 6% |
| Question 9. When should follow-up at PC level be considered? |
| - Patient in clinical remission with adequate growth and development, negative serology, adequate adherence to GFD and absence of special situations* |
| Agreement: 100% |
| Question 10. How should it be organised? |
| - Frequency: every 1−2 years, unless there is evidence of poor adherence to the diet |
| - Assess: signs and symptoms, growth and pubertal development, specific serology for CD and adherence to the diet |
| - In select cases, perform measurement of micronutrient levels, liver and/or thyroid function tests and a bone density scan |
| - Consider assessment of quality of life |
| - In patients followed up at the PC level, consider referral to a paediatric gastroenterology unit in the case of: recurrence of symptoms or development of new symptoms, problems with the diet, development of comorbidities or positive serology in the absence of known dietary transgressions |
| Agreement: 93%. Abstention: 7% |
| Question 11: What would be the approach to follow-up in PC of patients that do not keep their follow-up paediatric gastroenterology appointments? |
| - In these cases, assess for risk factors that would require hospital-based follow-up and whether follow-up assessments could be performed in PC |
| Agreement: 89%. Abstention: 7%. Disagreement:4% |
In patients who have not showed up to paediatric gastroenterology appointments for different reasons, assess whether hospital-based follow-up is necessary, based on the conditions previously discussed, or whether these check-ups can be performed in PC. The approach will vary depending on the level of knowledge and resources of the PC team, which may make the decision to refer the patient back to the paediatric gastroenterology unit. Efforts should be focused on patients with special situations requiring expert followup by a paediatric gastroenterologist and patients who repeatedly miss follow-up appointments, and, in the latter case, it may be helpful to report the situation to social work services.
What has been discussed in this section is the basis for the recommendations summarised in Table 3.
Section 3. Follow-up of adolescents (14–18 years)Question 12. Which providers should be involved?Adolescence is a stage of development characterised by the exploration of identity and a desire for increased autonomy. Since CD is a chronic disease that can only be controlled through diet, which does not require medication and which frequently remains asymptomatic even if adherence is not strict, adequate monitoring of CD is difficult in this period. This is compounded by changes in the health care providers who are responsible for the follow-up.
In most autonomous communities in Spain, in the PC setting, the care of patients is transferred from paediatricians to family physicians at age 14. In the hospital setting, the transition to adult care tends to occur later (age 16–18 years). Therefore, follow-up would continue to be multidisciplinary (section 2), but now involving family doctors and adult gastroenterologists.4,33–35
Question 13. How should it be organised?The same recommendations presented in previous sections apply.
Question 14. How should the transition to adult care be performed?The goal of the care transition process is to switch from a family-centred approach to a patient-centred approach in the management of CD.
Less than a third of adolescents with CD are followed up in adult gastroenterology settings,36,37 although a majority can be followed up exclusively at the PC level.37 On the other hand, at least one third of European paediatric gastroenterologists report the absence of care transition protocols in the centres where they work,38 which highlights the need to improve the transition to adult care in patients with CD.
Appropriate age for transition to adult careAccording to experts, the paediatrician should start discussing the transition at age 12–13 years, develop the transition plan at 14–15 years and implement it at 18 years, although age does not seem to influence subsequent adherence to the GFD.36 This plan may be subject to variations depending on local health care organisation, the level of disease activity, the degree of adherence to the diet and the autonomy of the patient. In patients with delayed puberty, deferring transition until puberty is complete is recommended. In Spain, the transition will probably take place earlier if the patient is followed up in the paediatric PC setting compared to a paediatric gastroenterology unit.
Factors that may affect transition outcomesThe predictors of successful transition include long-term adequate adherence to the GFD, diagnosis before age 12 years, regular follow-up and clinical remission and negative serology before 18 years. Poor adherence to the diet is associated with lack of follow-up, having T1D and economic difficulties in relation to the GFD. The current approach is to individualise the transition to adult care and the followup in adulthood taking into account the factors mentioned aboves.39
Although the use of emerging technologies, e-health and telehealth is currently infrequent in the follow-up of patients with CD, paediatricians consider them an adequate alternative to in-person visits for patients in remission.37,40
Question 15. What is the appropriate setting for the transition of care?Patients in follow-up in the PC paediatrics setting will transition to a family physician. Adolescents who have required follow-up in a specialised paediatric gastroenterology unit should be referred to an adult gastroenterology practice. All professionals involved should take into account the suggestions listed in Table 4 to achieve a successful transition.
Suggestions for the transition to adult care of adolescents with CD.
| 1. The primary care paediatrician or paediatric gastroenterologist should: |
|---|
| - Ensure that the diagnosis of CD has been made correctly |
| - Ensure that the patient understands the disease, knows how to follow the GFD correctly and the potential consequences of nonadherence (bone disease, infertility, risk of cancer, etc) |
| - Prepare the patient for the transition to adult care, which should not be abrupt |
| - Formally refer the patient by scheduling an adult care visit, either with a family physician or adult gastroenterology services, performing a last follow-up workup before referral |
| - Produce a report including all the necessary information to ensure the adult care provider can understand the medical history of the patient prior to transition and the patient’s current clinical condition |
| 2. PC paediatricians, paediatric gastroenterologists, family physicians and adult gastroenterologists should: |
| - Facilitate the performance of transition visits: joint visits with the patient and family or meetings of the different involved providers to introduce the patient |
| 3. The family physician and adult care gastroenterologist should: |
| - Consider CD as a condition similar to other severe chronic diseases, devoting the necessary attention to the adherence to the diet and the assessment of complications |
| - Establish positive rapport with the patient to ensure the adolescent does not feel judged when disclosing possible dietary transgressions |
| - Facilitate discussion of issues unrelated to CD (sexual activity, fertility, contraception, alcohol, drugs and psychological aspects) |
It is especially important that either the paediatric PC paediatrician or the paediatric gastroenterologist make a clinical report describing the characteristics of the patient from diagnosis to the time of transition (Table 5), so that the adult care physician receiving the patient gets a quick, clear and comprehensive overview of the patient’s medical history (Table 5).
Information to be included in the care transition report.
| 1. Demographic characteristics of the patient |
| 2. Date and age at diagnosis |
| 3. Clinical features at diagnosis/risk group assessment |
| 4. IgA level and serology at diagnosis |
| 5. Results of intestinal biopsy (if performed) |
| 6. Results of genetic testing (if performed) |
| 7. Results of bone scan (if performed) |
| 8. Associated diseases or comorbidities (if applicable) |
| 9. Date of initiation of gluten-free diet |
| 10. Adherence to gluten-free diet |
| 11. Evolution of growth and development, height and weight gain history |
| 11. Serology values throughout the follow-up |
| 11. Findings of intestinal biopsy during follow-up (if performed) |
| 14. Symptoms at the time of transition |
| 15. Serology at time of transition |
| 16. Family study (if performed) |
What has been discussed in this section is the basis for the recommendations summarised in Table 6.
Recommendations for the follow-up of adolescents (14–18 years).
| Question 12. Which providers should be involved? |
| - Multidisciplinary follow-up: paediatric gastroenterologist, PC paediatrician, family physician, adult gastroenterologist, nutritionist and/or specialised nurses |
| Agreement: 96%. Abstention: 4% |
| Question 13. How should it be organised? |
| - Apply recommendations for CD in remission (Table 2) |
| Agreement: 100% |
| Question 14. How should the transition to adult care be performed? |
| - Consider starting preparing for transition from age 12−13 years and implementing it between 15 and 18 years depending on the care setting where the patient was followed up and individual circumstances |
| - Personalise the transition to adult care considering factors that could hinder successful transition* |
| - Consider the use of emerging technologies in the follow-up of adolescents and young adults with stable CD |
| Agreement: 89%. Abstention: 11% |
| Question 15. What is the appropriate setting for the care transition? |
| - Patients in follow-up in the PC paediatrics setting will transition to a family physician. Adolescents who have required follow-up in a specialised paediatric gastroenterology unit should be referred to an adult gastroenterology practice. |
| Agreement: 85%. Abstention: 11%. Disagreement: 4% |
The authors have no conflicts of interest to disclose.
The remaining authors are detailed below:
Miriam Blanco. Department of Paediatrics, Hospital Universitario Fundación Jiménez Díaz, Madrid, Spain.
Carmen Miranda. Paediatric Gastroenterology, Hospital General Universitario Gregorio Marañón, Madrid, Spain.
Raquel Vecino. Paediatric Gastroenterology and Nutrition Unit, Hospital Clínico San Carlos, Madrid, Spain.
Javier Eizaguirre. Paediatric Gastroenterology, Hospital Universitario de Donostia, Donostia, Spain.
Salvador García Calatayud. Paediatric Gastroenterology, Hospital Universitario Marqués de Valdecilla, Santander, Spain.
Mercedes Juste. Paediatric Gastroenterology, Hospital Vistahermosa HLA, Alicante; Spain.
Felix Sánchez Valverde. Department of Paediatrics, Hospital Universitario de Navarra, Navarrabiomed, Pamplona, Spain.
Antonio Guardiola. Department of Digestive Disease, Hospital Universitario de Fuenlabrada, Fuenlabrada, Madrid, Spain.
Xavier Díaz; Collbató-El Bruc Outpatient Clinic, Esparraguer, Barcelona, Spain.
Carmen Ribes. Coeliac Disease and Immune Gastrointestinal Disease Unit, Instituto de Investigación Sanitaria La Fe, Valencia, Spain (Reviewer).
Isabel Polanco. School of Medicine, Universidad Autónoma de Madrid, Hospital Universitario Infantil La Paz, Madrid, Spain (Reviewer).
Appendix A presents the rest of the authors of the manuscript who are members of the Sociedad de Gastroenterología, Hepatología y Nutrición Pediátrica (SEGHNP), societies of paediatric primary care (AEPap and SEPEAP), Sociedad Española de Enfermedad Celíaca (SEEC), societies of adult gastrointestinal disease (AEG and SEPD) and societies of adult primary care (SEMFYC, SEMG and SEMERGEN).