Off-label drug use is a common practice in paediatrics. The aim of the present study was to estimate the knowledge of Spaniard paediatricians on off-label use.
Materials and methodsCross-sectional, multicentre, descriptive and national study from July 2012 to March 2013 using an on-line questionnaire on off-label use in children. An e-mail was sent to paediatricians who were members of the Spanish Association of Paediatrics (AEP) or its Regional or Paediatric Specialties Societies.
ResultsOut of 673 responses received, 75.1% of Spanish paediatricians knew the meaning of off-label use, 61% of them prescribed medicines outside the conditions authorised in their Summary of Product Characteristics (SPC) and 47% knew of the importance of noting the off-label use in the medical record. However, just under half of paediatricians informed parents, and only 22% wrote it down in the medical record.
ConclusionsMost Spanish paediatricians do not meet current regulations regarding off-label use. This regulation demands: justifying the decisions when off-label use is needed, and to write down in the medical record that, at least an oral consent from the parents has been obtained. This study reveals a fact that Spanish paediatricians must change. Meanwhile, it is a priority to continue with the implementation of consensus and clinical guidelines, to obtain more data on the efficacy and safety of off-label drug use in children, and to incorporate them into the SPC.
Un número elevado de fármacos se prescriben en niños bajo condiciones no autorizadas en ficha técnica (uso off-label). El objetivo principal del estudio es estimar el conocimiento sobre el uso de fármacos fuera de ficha técnica por parte de los pediatras españoles.
Material y métodosEstudio transversal, multicéntrico, descriptivo, de ámbito nacional, mediante encuesta on-line, enviada por correo electrónico a pediatras socios de la Asociación Española de Pediatría o de sus sociedades de Especialidades y Regionales, entre julio del 2012 y marzo del 2013.
ResultadosSe recibieron 673 respuestas. Un 71,5% de los pediatras españoles conocen el significado del término off-label, el 61% afirma que prescribe fármacos con indicaciones fuera de ficha técnica y un 47% conoce que dicho uso debe quedar reflejado en la historia clínica. Sin embargo, algo menos de la mitad informa a los padres y solo el 22% lo deja anotado en la historia clínica.
ConclusionesMuchos pediatras no cumplen con la normativa actual con respecto al uso de fármacos en condiciones no autorizadas en ficha técnica. Esta normativa exige: justificar las decisiones de uso off-label y registrar en la historia clínica que se ha obtenido el consentimiento, al menos verbal, de los padres. Se pone de manifiesto una realidad que los pediatras españoles deben cambiar. Mientras tanto, es prioritario continuar con la realización de documentos de consenso y guías de práctica clínica para ampliar la información sobre la eficacia y seguridad de los usos off-label en niños, y poder incorporarlos a las fichas técnicas autorizadas.
In Spanish Royal Decree 1015/2009, regulating the availability of drugs in special situations, “off-label” medicines are defined as “drugs used in conditions other than those included in the authorised summary of product characteristics”. The use of medications in such conditions “shall be exceptional and limited to situations in which there is a lack of authorised alternative treatments for a particular patient”. In addition, “the doctor must properly justify the need to use the drug in the patient's clinical history and must inform the patient/guardians of the possible benefits and potential risks, obtaining their written consent”.1
A large number of medications are prescribed in paediatric practice in conditions not authorised in the summary of product characteristics (SPC). The term “exceptional”, applied to off-label use, implies an exception to the norm, not infrequent use. The percentage varies according to the different areas of specific training in paediatrics, but it ranges between 20 and 80% of all prescriptions.2–13 Despite the publications and conference papers that have analysed the use of drugs in off-label conditions, and despite the measures adopted by health authorities, medicines agencies, international paediatric networks, associations and societies, paediatricians’ real knowledge of the use of drugs outside the terms of the SPC is limited. The result of this is that parents are frequently not informed that their child is going to receive a medication whose use is not authorised in the SPC, and they are not informed because paediatricians are unaware of the administrative situation of the drugs they prescribe. It is advisable to have guidelines or protocols that support the use of the drug in children with the scientific evidence available, even if the conditions of authorisation are different. This ensures that doctors act in accordance with good clinical practice.
The main objective of the OL-PED study is to estimate the current state of knowledge on off-label use of drugs among Spanish paediatricians and to assess the need to adopt measures to improve this situation so as to ensure better clinical practice. Secondary objectives are to estimate how well informed they are about legal liabilities arising from off-label use of medications and to ascertain the sources paediatricians use to obtain information on drugs.
Materials and methodsA cross-sectional, multicentre, descriptive, national study. Source of information for the study: data collection by anonymous, voluntary, multiple-choice on-line survey (Table 1), sent by e-mail to paediatricians who were members of the AEP (Spanish Association of Paediatrics), as well as to those who were members of the Specialised and Regional associations federated with the AEP. The survey was designed using Google Docs® technology.
Questionnaire for the OL-PED survey.
| Sex: male/female |
| Age: ≤29/30–39/40–49/50–59/60–69/≥70 |
| Year of graduation in Medicine: (free text) |
| Year of qualification as a specialist in Paediatrics: (free text) |
| Field in which you currently pursue your professional activity: Primary Care/Specialist Care |
| Autonomous community where you currently pursue your professional activity (drop-down menu) |
| Indicate your subspecialty (drop-down menu) |
| Indicate the response that in your opinion best describes an OL drug: |
| A drug prescribed for compassionate use/A drug prescribed in conditions other than those included in the SPC/A drug with no available SPC/A drug whose patent has expired/I am not aware of the existence and/or meaning of OL drugs |
| What percentage of your prescriptions do you think are written in OL conditions?: |
| None/Some/Many/All/Don’t know |
| When you prescribe an OL drug, do you note it in the medical record that the parents have been informed? |
| I am not aware of when I prescribe an OL drug/I never prescribe OL drugs/I prescribe OL drugs and I inform the parents but I do not note it in the medical record/I prescribe OL drugs, I inform the parents and I note it in the medical record/Other (free text) |
| If the child suffered unforeseen harmful effects through taking an OL drug, what factors do you think would support your action from the point of view of medical liability? |
| To have obtained the written consent of the parents in every case/To have informed the parents verbally, to have made a note in the medical record and to have protocols or clinical practice guidelines that endorse the action/To have protocols or clinical guidelines that endorse the action, without the need to record the fact that the parents have been informed, since scientific evidence exists/None of the above/Don’t know |
| What sources of information do you use to obtain information on drugs? (Never/Sometimes/Always) |
| Pharmaceutical industry information/Protocols or clinical guidelines/SPC of the drug/Vademecums or drug guides/Scientific journals/Other doctors/Sponsored meetings/Medical conferences |
| Do you consider it important to have a good knowledge of OL drugs for your normal clinical practice? (Score from 1 to 5, with 1 as completely unimportant and 5 as of the highest importance) |
OL: off-label.
The surveys were carried out between 1 July 2012 and 31 March 2013. Inclusion criteria: possession of a medical degree, status as specialist in Paediatrics or resident in training in this field (MIR), and membership of the AEP or of its federated Specialised or Regional associations.
The number of members of the AEP as of 1 July 2012 was 9754 paediatricians. E-mail addresses were available for 6027. In addition, approximately 3000 e-mails containing a direct link to the survey were sent to paediatricians in Specialised and Regional associations federated with the AEP. It was estimated that 800 paediatricians would reply, a sample of sufficient size to be able to estimate the current state of knowledge on off-label use of drugs among Spanish paediatricians, with an error of less than 3.2%, a level of homogeneity of 50% and a 95% confidence interval (CI), assuming that the total number of paediatricians in Spain is 10,000.
We collected data on the following: sex, age, year of graduation in Medicine, year of qualification as specialist in Paediatrics, field and autonomous community in which the respondent pursues his/her professional activity, area of paediatric subspecialties and responses to questions posed in the survey.
For the descriptive analysis of the qualitative variables we calculated the various relative frequencies. We performed a heterogeneity analysis using the χ2 test or the Fisher exact test for expected frequencies of less than 5. The data were analysed using the SPSS statistical program v19.0.
Given that patient data were not included and that the surveys were anonymous and voluntary, approval by an Ethics Committee was not considered necessary. Nevertheless, the Committee of the institution which organised the study was informed that it was being carried out and approval was obtained for the approach adopted. The study investigators were the only ones with access to the survey data, collected solely for statistical purposes.
ResultsWe received 673 responses. Of those surveyed, 69% were women. The majority of the paediatricians were aged between 30 and 49 (53.5%), followed by those over 50 (42.4%) and those under 30 (4.1%). This matches the time since graduation, which was between 20 and 39 years in 56% of cases. Of all respondents 3.6% were MIR and 28% had been specialists in Paediatrics for less than 10 years; 39% worked in Specialist Care and the rest in Primary Care. In terms of autonomous communities, those with the highest representation were Madrid (23%), Catalonia (14%), the Valencian Community (12%) and Andalucía-Ceuta and Melilla (8%), with descending percentages for the rest, although all were represented. As regards specific specialties, the answer from 37% was Primary Care, followed by General Paediatrics (21%) and Neonatology (6%), with percentages of between 1 and 4.5% for the remaining paediatric subspecialties, all of which were represented.
The responses to the questions posed in the survey are shown, differentiating between Primary and Specialist Care, in Tables 2 and 3.
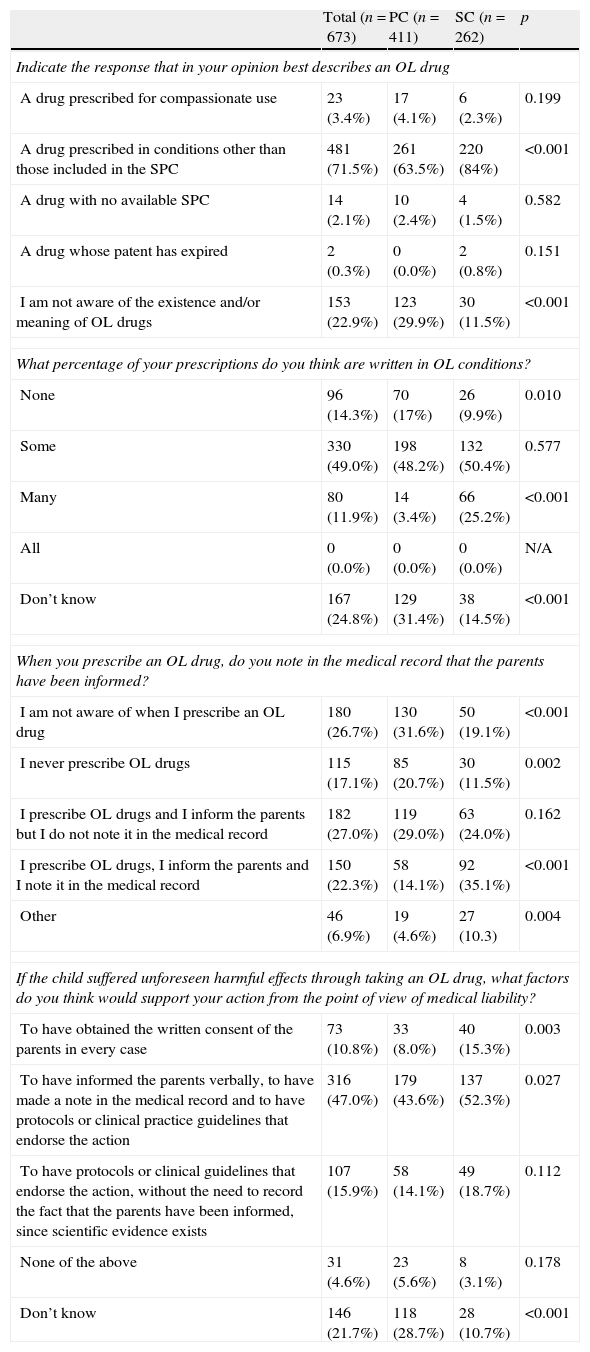
Responses to the OL-PED survey (part 1).
| Total (n=673) | PC (n=411) | SC (n=262) | p | |
| Indicate the response that in your opinion best describes an OL drug | ||||
| A drug prescribed for compassionate use | 23 (3.4%) | 17 (4.1%) | 6 (2.3%) | 0.199 |
| A drug prescribed in conditions other than those included in the SPC | 481 (71.5%) | 261 (63.5%) | 220 (84%) | <0.001 |
| A drug with no available SPC | 14 (2.1%) | 10 (2.4%) | 4 (1.5%) | 0.582 |
| A drug whose patent has expired | 2 (0.3%) | 0 (0.0%) | 2 (0.8%) | 0.151 |
| I am not aware of the existence and/or meaning of OL drugs | 153 (22.9%) | 123 (29.9%) | 30 (11.5%) | <0.001 |
| What percentage of your prescriptions do you think are written in OL conditions? | ||||
| None | 96 (14.3%) | 70 (17%) | 26 (9.9%) | 0.010 |
| Some | 330 (49.0%) | 198 (48.2%) | 132 (50.4%) | 0.577 |
| Many | 80 (11.9%) | 14 (3.4%) | 66 (25.2%) | <0.001 |
| All | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | N/A |
| Don’t know | 167 (24.8%) | 129 (31.4%) | 38 (14.5%) | <0.001 |
| When you prescribe an OL drug, do you note in the medical record that the parents have been informed? | ||||
| I am not aware of when I prescribe an OL drug | 180 (26.7%) | 130 (31.6%) | 50 (19.1%) | <0.001 |
| I never prescribe OL drugs | 115 (17.1%) | 85 (20.7%) | 30 (11.5%) | 0.002 |
| I prescribe OL drugs and I inform the parents but I do not note it in the medical record | 182 (27.0%) | 119 (29.0%) | 63 (24.0%) | 0.162 |
| I prescribe OL drugs, I inform the parents and I note it in the medical record | 150 (22.3%) | 58 (14.1%) | 92 (35.1%) | <0.001 |
| Other | 46 (6.9%) | 19 (4.6%) | 27 (10.3) | 0.004 |
| If the child suffered unforeseen harmful effects through taking an OL drug, what factors do you think would support your action from the point of view of medical liability? | ||||
| To have obtained the written consent of the parents in every case | 73 (10.8%) | 33 (8.0%) | 40 (15.3%) | 0.003 |
| To have informed the parents verbally, to have made a note in the medical record and to have protocols or clinical practice guidelines that endorse the action | 316 (47.0%) | 179 (43.6%) | 137 (52.3%) | 0.027 |
| To have protocols or clinical guidelines that endorse the action, without the need to record the fact that the parents have been informed, since scientific evidence exists | 107 (15.9%) | 58 (14.1%) | 49 (18.7%) | 0.112 |
| None of the above | 31 (4.6%) | 23 (5.6%) | 8 (3.1%) | 0.178 |
| Don’t know | 146 (21.7%) | 118 (28.7%) | 28 (10.7%) | <0.001 |
SC: Specialist Care; PC: Primary Care; OL: off-label.
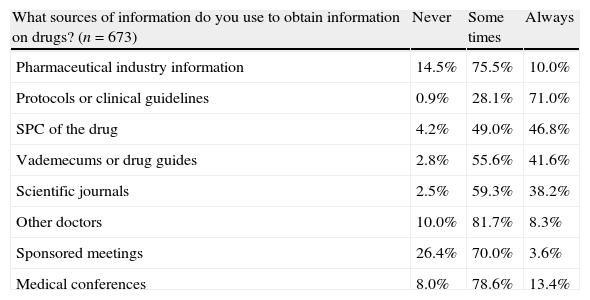
Responses to the OL-PED survey (part 2).
| What sources of information do you use to obtain information on drugs? (n=673) | Never | Some times | Always |
| Pharmaceutical industry information | 14.5% | 75.5% | 10.0% |
| Protocols or clinical guidelines | 0.9% | 28.1% | 71.0% |
| SPC of the drug | 4.2% | 49.0% | 46.8% |
| Vademecums or drug guides | 2.8% | 55.6% | 41.6% |
| Scientific journals | 2.5% | 59.3% | 38.2% |
| Other doctors | 10.0% | 81.7% | 8.3% |
| Sponsored meetings | 26.4% | 70.0% | 3.6% |
| Medical conferences | 8.0% | 78.6% | 13.4% |
The results of our survey show that 7 out of 10 Spanish paediatricians knew the meaning of the term “off-label” when applied to the specific use of a particular medicine. In addition, almost half of paediatricians, regardless of their professional field, stated that they have prescribed drugs with indications outside the authorised SPC. The fundamental difference between Primary and Specialist Care shows up in the other half of those surveyed: whereas the former never prescribe them or are unaware of when they do so, as many as 25% of paediatricians in Specialist Care reported that they prescribe them on many occasions.
The uncertainty of Spanish paediatricians regarding the off-label use of drugs increased as the survey proceeded. In the third question, on how to document in the medical record that the parents have been informed of off-label use of a particular medicine, approximately 50% of paediatricians maintained that they never prescribe drugs with this kind of use, or that they are unaware of it, or even that they administer them but do not report the fact. This percentage is significantly larger in Primary than in Specialist Care. The other half stated that they do know when they are engaging in this use and do inform the parents, but only 2 out of every 10 paediatricians document it in the medical record.
In the event of adverse reactions arising after administration of a medicine in unauthorised circumstances, the majority response of paediatricians in support of their actions was the fact that the parents have been verbally informed and that this use was noted in the medical record, as well as having protocols or clinical practice guidelines that endorse their actions. Almost 5 of every 10 paediatricians consulted were clear about this, but only 2 of every 10 stated that they report off-label use of drugs in writing in the record.
Finally, the importance paediatricians ascribe to knowledge of off-label use of drugs in their daily clinical practice, taking a score of 1 as completely unimportant and 5 as of the highest importance, was 3.92 (standard deviation 1.09). There were no significant differences in this score related to the professional field of work.
DiscussionThe information available in the SPCs of paediatric drugs is often insufficient, incomplete and inconsistent or contradictory.14,15 In most cases there is no dosing by weight, body area or age group.7,14 SPCs should be amended or completed according to the scientific evidence available at any time, in order to meet the therapeutic needs of a child population which is still at a disadvantage in the use of drugs compared with the adult population.14,16
At the end of the twentieth century the real situation of medicines in children was revealed. The European Union, following earlier experience in the United States, enacted a set of paediatric rules in 2007 with the intention of improving the quality of paediatric clinical trials and facilitating the availability of information in the SPCs of the drugs used in children.7,17 In addition, international networks of excellence have been developed within the European Union's Marco Programme, such as the Task-force in Europe for Drug Development for the Young (TEDDY), which have provided the European Medicines Agency with advice and documentary evidence on paediatric drugs18 and have promoted new research projects in Europe in this field.
Our study is limited and possibly biased in several respects. Firstly, our recruitment method may involve a selection bias, since willingness to respond by e-mail may reflect a specific type of paediatrician. Moreover, the paediatricians’ responses were trusted, and there may be differences between what they say and what they actually do. Finally, although the number of responses was slightly lower than we had hoped, it was a large enough sample size to estimate the current degree of knowledge on off-label use of medicines among Spanish paediatricians with an error of less than 3.65%, with the same conditions referred to above in the ‘Materials and methods’ section.
However, despite these limitations, we consider that the results of the survey reflect a current situation in which two phenomena exist regarding off-label use of drugs in Paediatrics. On the one hand, during the last 10–15 years many experts have been warning about off-label use of drugs in children, about the need to develop paediatric clinical trials and about the attitude pharmaceutical companies and health authorities ought to adopt on this issue. On the other, a substantial percentage of paediatricians continue prescribing medicines in their daily clinical practice without knowing whether or not the dose conforms to the SPC, and even without knowing whether or not the drug is indicated in children or at certain ages. Obviously if the paediatrician does not know, the family cannot be informed either. In any case, as things currently stand doctors treating children under 14 should know that the responsibility for off-label prescription of drugs still lies with the prescribing physician.
In Spain the Medicines Committee of the AEP (CM-AEP) was created to promote appropriate use of drugs for children in our country.18,19 On 17 December 2012 this Committee introduced the Pediamecum, a wide-ranging on-line database of medicines for paediatric use,20 also including details of which drugs are used in children in conditions other than those authorised in the SPC. Of the total of nearly 600 drugs available in this database, more than half have at least one recognised use in off-label conditions. These notably include medicines that are also regularly used in Primary Care, such as cyproheptadine, co-trimoxazole, dexchlorpheniramine, macrolides, mebendazole, metronidazole, omeprazole, racecadotril, ranitidine and rifampicin.19,20
Off-label use of medicines in Paediatrics is necessary and proper. Too often no authorised drugs exist for a particular therapeutic purpose, or those that do exist prove less appropriate for a specific patient. Paediatricians must be aware that the objective is the therapeutic benefit of the patient and must convey this to the parents. Similarly, they must justify the prescription in the light of existing scientific knowledge and clinical practice, make a note of it in the patient's medical record and obtain the verbal consent of the parents or legal guardians. Off-label use of a drug is legal, but it is not covered by the guarantees of Medicines Agencies, and if claims arise the drug companies are not liable.
It is also important to inform paediatricians and make them aware of the need to report any unforeseen harmful effect that occurs after using a drug as a suspected adverse drug reaction (ADR), using a yellow card or electronic form.21 Indeed, under current regulations, failure to report a suspected ADR could constitute a punishable offence.
In conclusion, our survey reveals that at the moment many paediatricians are not complying with current regulations regarding the use of drugs in conditions not authorised in the SPC. These regulations require them to distinguish between authorised and off-label uses, to account for off-label use decisions in the medical record and to record the fact that the parents have been provided with information on the treatment, as well as the fact that the parents have given their consent, at least verbally. Even though this is probably inconvenient for paediatricians, who are increasingly burdened with purely administrative work, our advice in the present situation is to comply with the current regulations. Meanwhile, it is a matter of priority to continue producing protocols, evidence analyses and clinical practice guidelines (the sources most commonly used by paediatricians to obtain information on medicines) with more extensive information on the effectiveness and safety of off-label uses in children, providing the scientific evidence available to support such uses and making it possible to incorporate them into the authorised SPCs.
Conflicts of interestDr María José Mellado Peña is the coordinator of the CM-AEP. Dr Lourdes Cabrera García and Dr Roi Piñeiro Pérez are members of the CM-AEP. The other authors have no conflicts of interest to declare.
Please cite this article as: Piñeiro Pérez R, Ruiz Antorán MB, Avendaño Solá C, Román Riechmann E, Cabrera García L, Cilleruelo Ortega MJ, et al. Conocimiento sobre el uso de fármacos off-label en Pediatría. Resultados de una encuesta pediátrica nacional 2012-2013 (estudio OL-PED). An Pediatr (Barc). 2014;81:16–21.
Study endorsed by the Medicines Committee of the Asociación Española de Pediatría (Spanish Association of Paediatrics) (CM-AEP).