Ultrasound has been used to quantify and qualify muscle morphology in critically ill children and can detect changes in muscle thickness. The aim of this study was to assess the reliability of ultrasound measurement of muscle thickness in critically ill children and to compare the assessments made by an expert with those made by inexperienced sonographers.
Material and methodsCross-sectional observational study conducted in the paediatric intensive care unit of a tertiary care university hospital in Brazil. The sample included patients aged 1 month to 12 years who received invasive mechanical ventilation for at least 24 h. Ultrasound images of the biceps brachii/brachialis and quadriceps femoris were obtained by one experienced sonographer and several inexperienced sonographers. We assessed intrarater and inter-rater reliability by means of the intraclass correlation coefficient (ICC) and Bland-Altman plot analysis.
ResultsMuscle thickness was measured in 10 children with a mean age of 15.5 months. The mean thickness of the assessed muscles as 1.14 cm for the biceps brachii/brachialis (standard deviation [SD], 0.27) and 1.85 cm for the quadriceps femoris (SD, 0.61). The intrarater and inter-rater reliability were good for all sonographers (ICC > 0.81). The differences were small, there was no significant bias in the Bland-Altman plots and all measurements were within the limits of agreement, except for 1 measurement of biceps and quadriceps.
ConclusionSonography can be used in critically ill children to accurately assess changes in muscle thickness, even by different evaluators. More studies are needed to establish a standardised approach to the use of ultrasound for monitoring muscle loss in order to incorporate it in clinical practice.
La ecografía se ha utilizado para cuantificar y calificar la morfología muscular de niños críticamente enfermos, detectando posibles cambios en el grosor muscular. El objetivo del estudio fue evaluar la fiabilidad de la medición por ecografía del grosor muscular en niños críticamente enfermos y comparar la evaluación de un examinador experto con la de examinadores con poca experiencia.
Material y métodosEstudio observacional transversal en la unidad de cuidados intensivos pediátricos de un hospital universitario de tercer nivel en Brasil. Se incluyeron pacientes entre 1 mes y 12 años que recibieron ventilación mecánica invasiva durante un mínimo de 24 horas. Se obtuvieron imágenes ecográficas del bíceps braquial/braquial y cuádriceps femoral en evaluaciones realizadas por un ecografista experimentado y ecografistas inexpertos. La concordancia intra- e interevaluador se estableció mediante el coeficiente de correlación intraclase (CCI) y el análisis gráfico de Bland-Altman.
ResultadosSe midió el grosor muscular en 10 niños con una edad media de 15,5 meses. El grosor medio de los músculos evaluados fue de 1,14 cm ± 0,27 para el bíceps braquial/braquial y de 1,85 cm ± 0,61 para el cuádriceps femoral. La fiabilidad intraevaluador e interevaluador fue muy buena (CCI > 0,81) para todos los ecografistas. Las diferencias fueron pequeñas, sin detectarse en el análisis de los gráficos de Bland-Altman, y todas las mediciones estuvieron dentro de los límites de concordancia, excepto una medición de bíceps y cuádriceps.
ConclusiónLa ecografía se puede utilizar en niños en estado crítico para evaluar con precisión los cambios en el grosor muscular, incluso por diferentes evaluadores. Se necesitan más estudios para establecer un enfoque estandarizado en el uso de esta herramienta para la monitorización de la pérdida muscular con el fin de incorporar su uso en la práctica clínica.
Hospitalization in the intensive care unit (ICU) is associated with several complications, including muscle weakness, commonly associated with an increase in morbidity and mortality in both adults and children.1,2 Intensive care unit-acquired weakness (ICU-AW) is a condition diagnosed following critical illness and it is important to differentiate it from other acute neuromuscular diseases that can cause respiratory failure and ICU admission.3,4 Moreover, ICU-AW is symmetrical, diffuse, and generalized, involving all four extremities, and it is associated with muscle atrophy, hypotonia and hyporeflexia.3,5
Contrary to studies in the adult population, there is little research on ICU-AW in the paediatric population,6 and there is high variability in the reported incidence in this population, caused in part by the lack of knowledge about this condition and the challenges in detecting muscle weakness in critically ill children.7 However, it is already known that this condition is associated with longer hospitalization, which has an impact on the course of disease and functional recovery.4
Ultrasonography is a non-invasive technique that can be used to detect changes in muscle structure and morphology. It can be performed at the bedside and is easy to implement, inexpensive and available in most hospitals.8 It has been proposed as a possible tool for assessment of critically ill patients, as it allows early detection of muscle loss, which is associated with histological changes and functional decline.9 Therefore, it is a useful tool that can avoid the use of methods to study muscle changes, for instance, electrophysiological tests like electromyography (EMG) and nerve conduction study (NCS), which are more complex and expensive and are not available in many ICUs.10
Previous studies in the paediatric population have demonstrated that sonography can detect changes in muscle thickness in critically ill children, but it has only recently started to be used as an evaluation method.10,11 In consequence, many aspects are still unclear, such as the most suitable muscles for measurement, or the technique, duration, echogenicity, and reliability of muscular ultrasonography for clinical use in critically ill children. Thus, the purpose of our study was to evaluate the reliability of the sonographic measurement of the thickness of the biceps brachii/brachialis and quadriceps femoris muscles in critically ill children and to compare reliability in an expert examiner and newly trained examiners.
Materials and methodsStudy designWe conducted a cross-sectional observational study.
SampleParticipants were recruited between August and November 2021 in the paediatric intensive care unit (PICU) of a tertiary university hospital in Southern Brazil. We selected patients of both sexes, aged from 1 month to 12 incomplete years, with a parent or legal guardian, and who had received invasive ventilation for at least 24 h. We excluded patients who were ventilator-dependent prior to PICU admission, readmitted to the PICU within 24 h from discharge; with trauma in any of the extremities to be evaluated or with any other anatomical abnormalities that precluded an adequate muscle evaluation or with a diagnosis of with neuromuscular disease, cerebral palsy, stroke or any other neurological and/or genetic disease that manifests with muscle weakness and/or changes in muscle tone. We retrieved clinical and demographic information from the electronic health records, including the following: sex, age (months), diagnosis, reason for admission, weight, and stature.
The study was approved by the research ethics committee (2021-0119). Once approved, we started sample recruitment and had the parents and/or guardians of the participants sign an informed consent form (ICF), in adherence with Resolution 466/12 regulating research involving human subjects.
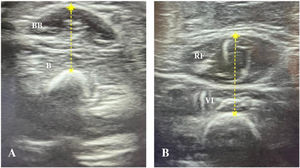
Ultrasound muscle assessment protocolSonographic assessments were performed with an InnoSight portable ultrasound device (Koninklijke Philips N.V., Netherlands) with a 4−12 MHz linear probe, applying the same imaging settings, such as time gain compensation, focus, and depth in all evaluations. The measurements were performed within 24 h of intubation, following the protocol described by Ng et al.,12 with assessment of two muscle groups in each child: right biceps brachii/brachialis and right quadriceps femoris (Fig. 1). If the right upper and lower limbs were not accessible due to some subjacent structure (eg peripheral venous catheter, external fixator), the contralateral side was evaluated. During the evaluation, patients were in the supine position, with arms and legs extended and muscles relaxed. The upper extremity was supinated and the lower extremity positioned with the knee extended and the ankle in a neutral position. Transverse ultrasound images of the muscles were obtained at predefined anatomical locations, measuring the distance from bony landmark references: a) biceps brachii/brachialis: 2/3 distance from the acromion to the antecubital fold; b) quadriceps femoris: 1/2 distance from the anterosuperior iliac spine to the upper border of the patella. A permanent marker was used to mark the location where the transducer was to be placed on the skin. During the evaluation, the transducer was placed perpendicular to the skin with a generous amount of contact gel, always with minimal pressure to avoid muscle compression.
The measurements were performed by four examiners, one expert examiner (a radiologist with experience in sonography), and 3 trained examiners (trained examiners 1, 2, and 3). The former was a certified radiologist with 2 years of experience in musculoskeletal radiology. The latter were physical therapists without previous experience in muscle imaging or muscle thickness measurement and had limited exposure to sonography. The training consisted of an initial bedside practice for all the trainees, held specifically for this study, which lasted 3 h and focused on the measurements of the 2 regions of interest. In the initial training, several measures were taken by each trainee. The expert examiner was available for additional training and clarifications as needed throughout the study period and assessed the competence of the other examiners in the bedside ultrasound training.
Each examiner performed 3 consecutive measurements using ultrasound in a single plane at the marked anatomical site. During training, the transducer placement site along the limb was marked by a single examiner to achieve greater interrater reliability. The initial marking was made by the first examiner, whether experienced or in training. All images were obtained when the patients were maximally relaxed, regardless of the level of consciousness, and patients were allowed to move or reposition if necessary.
Intrarater analysisFor each muscle, three consecutive muscle thickness measurements were obtained at a single time. The largest and smallest of the three measurements taken were used to analyse intrarater variability.
Interrater analysisThe examiners performed the measurements of the biceps brachii and quadriceps on the same day, preferably in the same shift. We calculated the mean of the 3 measurements by each examiner was calculated, and assessed the differences between the measurements of the newly trained examiners and the experienced one.
StatisticsThe statistical analysis was performed with the software SPSS Statistics 28.0 (IBM Corporation, Armonk, NY, USA), considering P values of less than .05 statistically significant. We expressed categorical variables as absolute frequencies and percentages, and continuous variables were expressed as mean and standard deviation (SD) or median and interquartile range depending on the distribution of the data. The normality of the data was assessed by means of the Kolmogorov-Smirnov or Shapiro-Wilk test.
Reliability was assessed by means of the intraclass correlation coefficient (ICC) using the two-way mixed effects model and by Bland-Altman plot analysis. We used the ICC to determine the level of agreement: poor (<0.21), fair (0.21−0.40), moderate (0.41−0.6), good (0.61−0.80) and very good (0.81–1.00). We calculated the limits of agreement as the mean difference ± 1.96 × SD between measurements, as previously described.12,13 We generated the plots to obtain a visual representation of the interrater agreement of the measurements and to identify potential systematic errors.
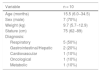
ResultsDuring the training period, 13 children underwent assessment of muscle thickness. Once data collection finished, the expert and the 3 trained examiners reviewed the obtained images and the expert considered 3 measurements (1 in each of 3 different patients) to be inadequate (too much pressure on the transducer or evaluation of muscle at an incorrect location). The decision was made by consensus to exclude inadequate measurements, leaving 10 patients in the study. Table 1 summarises the clinical and demographic data. The main reason for admission to the PICU was acute respiratory failure (50%), and the mean thickness of the assessed muscles was 1.14 cm (SD, 0.27) for the biceps brachii/brachii and 1.85 cm (SD, 0.61) for the quadriceps femoris.
Demographic and clinical characteristics of critically ill children at the time of admission to the PICU.
| Variable | n = 10 |
|---|---|
| Age (months) | 15.5 (6.0−34.5) |
| Sex (male) | 7 (70%) |
| Weight (kg) | 9.7 (5.7−12.9) |
| Stature (cm) | 75 (62−89) |
| Diagnosis: | |
| Respiratory | 5 (50%) |
| Gastrointestinal/Hepatic | 2 (20%) |
| Cardiovascular | 1 (10%) |
| Oncological | 1 (10%) |
| Metabolic | 1 (10%) |
Results expressed as median (interquartile range) or n (%).
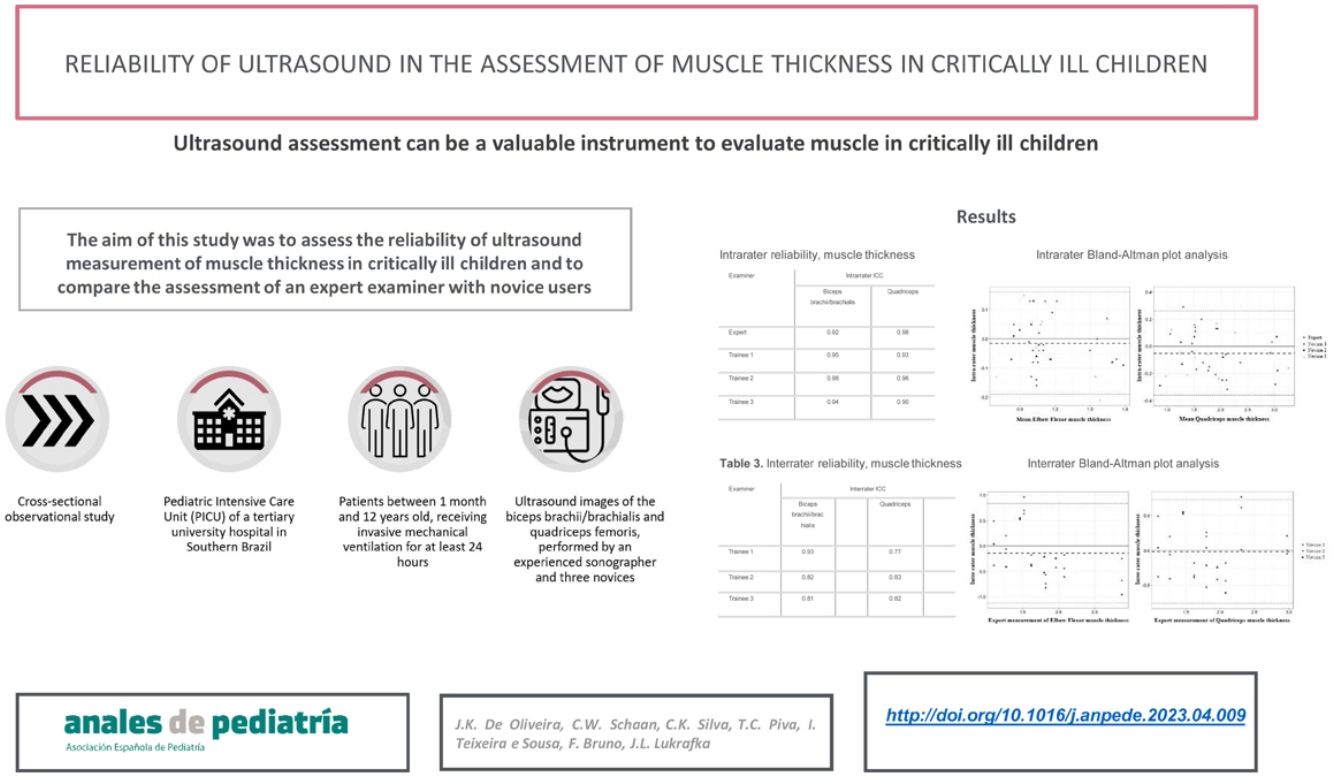
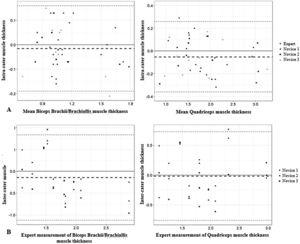
The intrarater reliability for the expert and inexperienced examiners was very good (ICC > 0.81) (Table 2). The ICC was similar among the examiners, and was higher for the lower extremity muscles in the expert assessment and for the upper extremity muscles among the trainees. The intrarater differences for the expert and inexperienced sonographers were small and did not exhibit bias in the analysis of the Bland-Altman plots (Fig. 2A). All obtained measurements were within the limits of agreement (−0.19 cm to 0.16 cm for the biceps brachii and −0.36 cm to 0.26 cm for the quadriceps), except for 1 quadriceps measurement by one of the evaluators. The mean difference between measurements was −0.01 cm for the upper limb muscles and −0.05 cm for the lower limb muscles.
Bland-Altman plots for expert and inexperienced examiners.
A: Intrarater Bland-Altman analysis of the differences between the repeated measures (cm) and the average muscle thickness (cm).
B: Interrater Bland-Altman analysis of the differences between the repeated measures (cm) and the expert measurements of muscle thickness (cm).
The interrater reliability (Table 3) was a little lower compared to the intrarater reliability, but still very good (ICC > 0.81), except for one of the inexperienced examiners in the quadriceps measurements. The differences between the examiners were small and showed no bias in the analysis of the Bland-Altman plots (Fig. 2B). All measurements obtained were within the limits of agreement (−1.12 cm to 0.83 cm for biceps and −0.76 cm to 0.72 cm for quadriceps), except for one brachii/brachialis biceps measurement and one quadriceps measurement by one of the evaluators. The mean difference was −0.14 cm for the upper extremity muscles and −0.01 cm for the lower extremity muscles.
DiscussionAt present, bedside ultrasound is considered a non-invasive muscle assessment technique that can be used to identify muscle changes in children at risk of developing muscle atrophy in the critical care setting. In our study, we observed that newly trained examiners could perform muscle ultrasound examinations even in highly complex care settings such as the PICU.
The sonographic evaluation of muscle thickness was accurately reproduced by single examiners and different examiners, with ICCs values for intrarater and interrater reliability considered to be very good, similarly to previous studies performed in children12,14,15 and critically ill adults.16 However, it is worth noting that the interrater coefficients were lower, although still very good, contrary to the findings of Ng et al.12
Before the ultrasound training, the inexperienced examiners had no experience with muscle imaging or muscle thickness measurement. Thus, the experienced examiner supervised and evaluated the measurements performed by the trainees, pointing out any irregularities and making the necessary corrections (position and pressure of the transducer on the skin, for example), thus minimizing potential measurement variability. Measuring the thickness of small muscles, for instance, in children aged less than 1 year (50% of the sample), was a challenge. Any small variation could mask a relative change in muscle thickness and decrease the reliability of the measurements.
Previous studies in critically ill children have demonstrated that the reliability of sonographic measurements of the quadriceps femoris muscle thickness is dependent on the number of images obtained.14,17 In our study, we used the technique described by Ng et al.,10 obtaining 3 transverse images of each evaluated muscle group instead of only one. So doing, we were able to repeat the measurements with differences of 7% or less between the evaluators in both muscles, producing more accurate and consistent results. Furthermore, our method differs from previous studies that only evaluated the quadriceps,17–19 as we also assessed the biceps brachii/brachialis to increase the reliability.
Currently, the quadriceps is the most studied muscle group for this type of evaluation,10 with measurement of its cross-sectional area and/or muscle thickness, the latter being the most frequently reported method in the literature.20 Studies in adults have demonstrated that sonography is a valid tool to detect qualitative and quantitative changes in muscle,21,22 with excellent intrarater and interrater reliability; however, there is no standard operational protocol for this measurement yet, which limits the widespread application of the technique.22,23 Moreover, in critically ill children, there is limited evidence supporting the use of sonography for detection of muscle wasting. To date, this method is not considered reliable in this population.10,14
Similar to the studies of Valla et al.17 and Ng et al.,12 our study protocol required marking the site of transducer placement on the evaluated muscle only once. If the markings happened to be erased, the measured distance between bony landmarks was used for subsequent measurements. Despite this, we managed to achieve a good reliability by measuring the area between the bony landmarks, even though we did not record the values for future reference. We suggest that in future studies, these values could be recorded to ensure greater reliability.
Our study has some limitations. First, some of our patients were not completely sedated or moved during the assessment. An increase in muscle tonus with movement or a change in the patient’s level of consciousness may alter the measured thickness, even though the examiners were trained to perform the measurements during periods of relaxation in awake and moving patients. We believe that this could be the reason for the measurements outside the limits of agreement in the interrater analysis. Secondly, some images showed changes in muscle echogenicity. These alterations, due to either acute or chronic disorders, may hinder the visualization of the ultrasound image and preclude an accurate assessment of muscle thickness. We suggest that future studies evaluate and investigate changes in echogenicity, such as changes in adipose and fibrous tissue, in association with muscle thickness measurements, providing a qualitative and quantitative analysis of the images obtained. Last of all, this study was conducted at a single centre and involved a small number of participants, which may limit its generalizability. In addition, we excluded children diagnosed with neuromuscular diseases or neurological disorders associated with muscle weakness or hypotonia, therefore, our results may not be applicable to these patients.
ConclusionUltrasound assessment can be a valuable instrument for accurate assessment of muscle thickness in critically ill children. In our study, we found a high reliability in the measurements of the biceps brachii/brachialis and quadriceps femoris, and high interrater reliability copmaring newly trained and experienced examiners. Muscle ultrasound might also be used as a tool for monitoring muscle mass loss, so accurate assessment methods are needed to detect small changes in muscle thickness throughout the stay in critical care units, especially in young children.
Further studies are required to establish a standard approach to sonography as a tool for monitoring muscle change in critically ill children and integrate its use in clinical practice in PICUs.
FundingThis research did not receive any specific grants from funding agencies in the public, private or not-for-profit sectors.
Ethical approvalThe study was approved by the Research Ethics Committee (2021-0119).
Conflicts of interestThe authors have no conflicts of interest to declare.