The objectives of this consensus document were to establish a standardized list of high-risk medications for the pediatric population and to compile the recommended practices for their safe use with the aim of promoting the implementation of medication error prevention programs in health care centers.
MethodThe Ministry of Health, the Spanish Institute for Safe Medication Practices, the Spanish Association of Pediatrics and the Spanish Society of Hospital Pharmacy and regional administration representatives participated in the project. The Recommendations for the Safe Use of High-Risk Medications was used as reference, and its contents adapted and expanded to address specific issues in the pediatric population based on the current evidence.
ResultsThe document provides a reference list of high-risk medicines in pediatric care. It recommends that health care centers, in addition to prioritizing interventions in relation to anticoagulants, insulins, opioids, neuromuscular blockers, IV potassium, oral methotrexate and cytostatic agents, also consider interventions for IV adrenergic agonists, aminoglycosides and vancomycin, drugs for moderate and minimal sedation, parenteral nutrition and IV paracetamol in pediatric patients. The document emphasizes the need to implement multiple safe practices at every stage of the medication use process, prioritizing those with the greatest effectiveness, and involving pediatricians, pharmacists and other healthcare professionals. It also highlights the importance of active involvement by patients and caregivers. Finally, it provides general guidelines common to all these medications, as well as specific practices for each prioritized pharmacological group or medication, which should be combined to enhance pediatric patient safety.
ConclusionDeveloping programs to increase the safety of high-risk medications in pediatric patients is essential in order to reduce medication errors in this vulnerable population. The implementation of safe practices should be accompanied by continuous monitoring and periodic updates to guarantee effectiveness and strengthen the safety culture in health care centers.
Los objetivos de este documento de consenso han sido establecer una lista estandarizada de medicamentos de alto riesgo en pediatría y recoger las prácticas recomendadas para su uso seguro, con el fin de promover la implementación de programas dirigidos a prevenir errores de medicación en los centros sanitarios.
MétodoEn su realización participaron el Ministerio de Sanidad, el Instituto para el Uso Seguro de los Medicamentos, la Asociación Española de Pediatría y la Sociedad Española de Farmacia Hospitalaria, así como representantes autonómicos. Se utilizó como referencia el documento de Recomendaciones para el Uso Seguro de los Medicamentos de Alto Riesgo, cuyo contenido se adaptó y amplió para abordar los problemas específicos de la población pediátrica, considerando la evidencia disponible.
ResultadosEl documento proporciona una lista de referencia de medicamentos de alto riesgo en pediatría. Recomienda que los centros sanitarios, además de priorizar las intervenciones en anticoagulantes, insulinas, opiáceos, bloqueantes neuromusculares, potasio IV, metotrexato oral y citostáticos, en pediatría consideren agonistas adrenérgicos IV, aminoglucósidos y vancomicina, medicamentos para sedación moderada y mínima, nutrición parenteral y paracetamol IV. Incide en la necesidad de implementar múltiples prácticas seguras en todas las etapas del circuito de los medicamentos, priorizando aquellas de mayor efectividad, contando con la participación de pediatras, farmacéuticos y otros profesionales sanitarios. También resalta la importancia de la participación activa de pacientes y cuidadores. Finalmente recoge prácticas generales comunes a todos estos medicamentos y prácticas específicas para cada grupo farmacológico o medicamento prioritario, que deben combinarse para mejorar la seguridad.
ConclusiónDesarrollar programas de mejora de la seguridad de los medicamentos de alto riesgo en pediatría es esencial para reducir los errores de medicación en esta población vulnerable. La implantación de prácticas seguras debe ir acompañada de un seguimiento continuo y de una actualización periódica para garantizar su efectividad y fortalecer la cultura de seguridad en los centros sanitarios.
According to the World Health Organization (WHO), medication errors continue to be one of the leading causes of preventable harm in health care systems worldwide. In fact, harm due to medicines may account for up to 50% of all preventable harm in medical care.1 To address this issue, in 2017 the WHO launched the third Global Patient Safety Challenge, Medication Without Harm,1 and subsequently adopted the Global Patient Safety Action Plan 2021–2030,2 which includes a strategic objective aimed at implementing practices to improve medication safety based on the recommendations of the third challenge. The Medication Without Harm initiative defined a strategic framework with three priority areas for action, one of which adresses medication safety in high-risk situations, which include the use of high-risk medications and the management of patients who are more vulnerable to medication errors, such as pediatric patients.3
High-risk or high-alert medications are drugs that bear a heightened risk of causing significant patient harm or even death when they are used in error. This is a key concept in patient safety that was introduced by the Institute for Safe Medication Practices (ISMP) to identify medicines on which to target safety efforts and improvement interventions.4 On the other hand, pediatric patients constitute a particularly vulnerable group on account of several factors that increase both the frequency of medication errors and the severity of the associated adverse events.5,6 Therefore, for this patient group, it is particularly crucial to implement effective measures to prevent errors in the use of these medications and to minimize the severe or fatal consequences that may result.1
In Spain, the Ministry of Health, in the framework of the Strategy for Patient Safety of the National Health System,7 has promoted the adoption of safety practices for high-risk medications and the development of specific interventions to reduce the incidence of medication errors in pediatric patients. In 2023, in collaboration with the ISMP-Spain, the Ministry of Health published the Recommendations for the Safe Use of High-Risk Medications,8 a document intended to help providers manage these medications safely in health care settings. The document noted that certain settings, such as pediatric care units, should adapt the already existing list of high-risk medications and add other medicines considered to carry a high risk for pediatric patients as well as the corresponding safety practices.
The objectives of this consensus document were to provide a reference list of high-risk medications for the pediatric population and present the practices recommended for their safe use in order to promote the implementation of programs for the prevention of medication errors in pediatric patients in health care centers in Spain.
MethodsThis document, which provides recommendations for the safe use of high-risk medications in pediatric patients, was developed through the collaboration of the Ministry of Health, the Instituto para el Uso Seguro de los Medicamentos (ISMP-Spain), the Asociación Española de Pediatría (AEP, Spanish Association of Pediatrics) and the Sociedad Española de Farmacia Hospitalaria (SEFH, Spanish Society of Hospital Pharmacists) as well as representatives from the autonomous communities of Spain. It began with the formation of a steering committee and a scientific committee composed of members of the participating institutions and societies. In addition, the AEP and the SEFH appointed a technical committee of drug safety experts composed of members of their respective working groups.
This document was developed using the Recommendations for the Safe Use of High-Risk Medications8 as reference, adapting and expanding its contents in consideration of the specific safety issues in the pediatric population. The development of the document was organized in several steps.
- -
Drafting the initial version of the document
The members of the scientific committee developed an initial draft with a list of high-risk medications in pediatric care, providing directions for the management of these drugs in health care organizations and general safety practices, and specifying the high-risk individual medications or drug classes for which interventions should be prioritized.
To this end, the committee used the existing document that provides general recommendations as well as information obtained through the review of the evidence available in the PubMed database and the websites of governmental agencies, safety organizations and scientific societies. The committee also considered the serious patient safety incidents documented in the reporting system of the ISMP-Spain and the Patient Safety Reporting and Learning System (SiNASP) of the Ministry of Health.
In parallel, the members of the expert committee developed safety practice recommendations for the prioritized high-risk medications, which were revised by the scientific committee and then added to the document to complete the initial draft. Lastly, the draft was revised by the Ministry of Health.
- -
Revision of the initial draft by the autonomous communities
The Ministry of Health submitted the initial draft to the autonomous communities for review by Patient Safety Strategy representatives, health care professionals and/or experts in medication safety.
- -
Development of the final version of the document
The scientific and steering committees analyzed the comments and proposed changes to the initial draft formulated by professionals from the different autonomous communities to produce the final version of the document.
ResultsThis article presents a summary of the resulting document, Recomendaciones para el Uso Seguro de los Medicamentos de Alto Riesgo en Pediatría, whose full text is available for consultation online.9
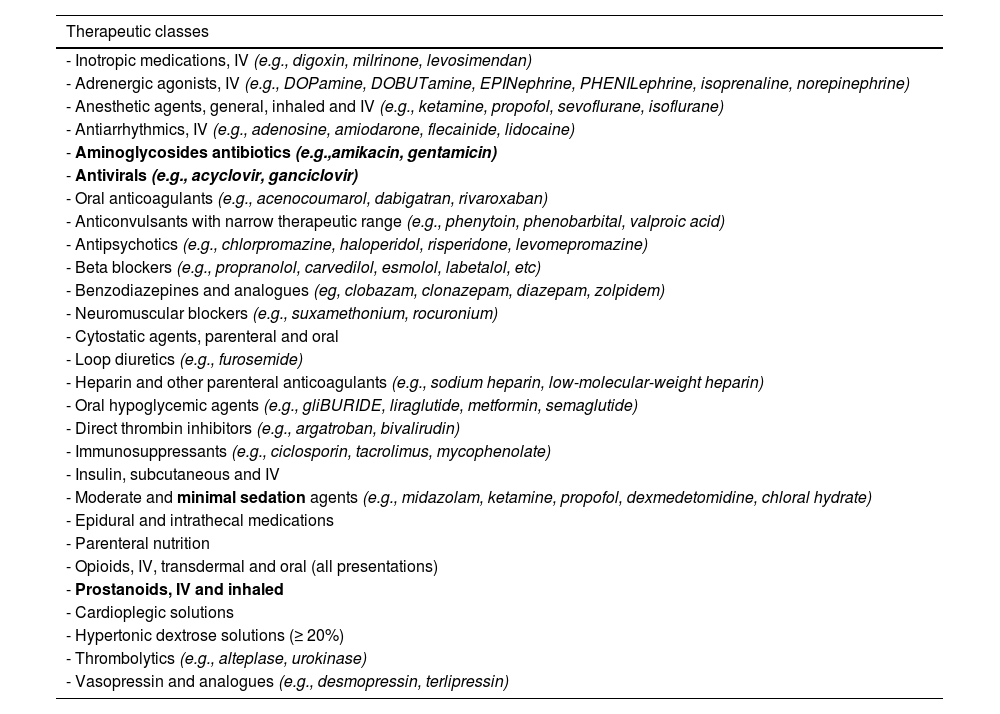
Section I. High-risk medication reference list for pediatric careThis document provides a high-risk medication reference list for pediatric patients (Table 1), which was developed taking into account the medications used in children featured in the high-risk medication reference lists for hospitals and chronic patients of the Recommendations for the Safe Use of High-Risk Medications document8 as well as specific lists for pediatric patients compiled through the review of the literature.10–15
High-risk medication list for pediatric patients.
| Therapeutic classes |
|---|
| - Inotropic medications, IV (e.g., digoxin, milrinone, levosimendan) |
| - Adrenergic agonists, IV (e.g., DOPamine, DOBUTamine, EPINephrine, PHENILephrine, isoprenaline, norepinephrine) |
| - Anesthetic agents, general, inhaled and IV (e.g., ketamine, propofol, sevoflurane, isoflurane) |
| - Antiarrhythmics, IV (e.g., adenosine, amiodarone, flecainide, lidocaine) |
| - Aminoglycosides antibiotics (e.g.,amikacin, gentamicin) |
| - Antivirals (e.g., acyclovir, ganciclovir) |
| - Oral anticoagulants (e.g., acenocoumarol, dabigatran, rivaroxaban) |
| - Anticonvulsants with narrow therapeutic range (e.g., phenytoin, phenobarbital, valproic acid) |
| - Antipsychotics (e.g., chlorpromazine, haloperidol, risperidone, levomepromazine) |
| - Beta blockers (e.g., propranolol, carvedilol, esmolol, labetalol, etc) |
| - Benzodiazepines and analogues (eg, clobazam, clonazepam, diazepam, zolpidem) |
| - Neuromuscular blockers (e.g., suxamethonium, rocuronium) |
| - Cytostatic agents, parenteral and oral |
| - Loop diuretics (e.g., furosemide) |
| - Heparin and other parenteral anticoagulants (e.g., sodium heparin, low-molecular-weight heparin) |
| - Oral hypoglycemic agents (e.g., gliBURIDE, liraglutide, metformin, semaglutide) |
| - Direct thrombin inhibitors (e.g., argatroban, bivalirudin) |
| - Immunosuppressants (e.g., ciclosporin, tacrolimus, mycophenolate) |
| - Insulin, subcutaneous and IV |
| - Moderate and minimal sedation agents (e.g., midazolam, ketamine, propofol, dexmedetomidine, chloral hydrate) |
| - Epidural and intrathecal medications |
| - Parenteral nutrition |
| - Opioids, IV, transdermal and oral (all presentations) |
| - Prostanoids, IV and inhaled |
| - Cardioplegic solutions |
| - Hypertonic dextrose solutions (≥ 20%) |
| - Thrombolytics (e.g., alteplase, urokinase) |
| - Vasopressin and analogues (e.g., desmopressin, terlipressin) |
| Specific medications |
|---|
| - Liposomal amphotericin B |
| - Calcium, IV (gluconate, chloride) |
| - Clonidine |
| - Potassium chloride, IV (concentrate) |
| - Hypertonic sodium chloride solution (> 0,9%) |
| - EPINephrine, IM and SC |
| - Potassium phosphates, IV |
| - Nitroprusside sodium, IV |
| - Paracetamol, IV |
| - Magnesium sulfate, IV |
| - Vancomycin |
Abbreviations: IM, intramuscular; IV, intravenous; SC, subcutaneous.
High-risk medications specific to the pediatric population that are not included in the reference lists provided in the Recommendations for the Safe Use of High-Risk Medications document are indicated in bold.8
This list should be used as a reference by health care organizations to produce their own high-risk medication lists for the pediatric population, which they will use for developing, prioritizing and implementing preventive measures (see Section II).
Section II. Recommendations for the management of high-risk medications in health care organizationsThe WHO and patient safety organizations emphasize the need for health care organizations to develop and implement programs to improve high-risk medication safety aimed at reducing errors at every stage of the medication use process.3,8,16–25 These programs must be an organizational priority and be supported and led at the institutional level in order to convey their importance to the entire organization.
The organization’s high-risk medication management program should be strengthened and expanded by taking into account the specific safety concerns associated with the pediatric population. This requires the engagement of pediatricians, pharmacists and other professionals involved in the care of this population, whose role is to develop initiatives that specifically target pediatric patients to be integrated in the general safety practices implemented by the organization and to ensure their implementation in clinical practice.
The measures that organizations need to take to develop a program to reduce errors involving high-risk medications are:
- 1
Develop an organization-specific list of high-risk medications to prioritize in the implementation of safety practices
An organization-specific high-risk medication list for pediatric patients should be developed using the general high-risk medication list presented in the previous section as a reference and taking into consideration the characteristics of the pediatric population served. The list should include, at a minimum, the following medications which are deemed high priority for all patient groups, including both adult and pediatric patients:
- -
Anticoagulants, insulins, opioids, neuromuscular blockers, IV potassium, oral methotrexate (non-oncologic use) and cytostatic drugs (unless they are not used at the facility).
In addition, the following should be included specifically for pediatric patients:
- -
IV adrenergic agonists, aminoglycosides and vancomycin, medications used for moderate and minimal sedation, parenteral nutrition and IV paracetamol.
Additional medications from the general reference list may be included, taking into account the most serious incidents recorded in the specific organization or identified in medication and patient safety publications. A key consideration is that the list should not be too long to ensure the feasibility of implementing the necessary safety practices in the organizations, as a list in itself is of little value if the health care staff is unaware of its existence or is not accompanied by effective risk-reduction strategies.16
- 2
Select and implement multiple safety practices at the various stages of the medication use process for each therapeutic class or specific medication included in the health care organization’s high-risk list
Each organization must select and implement multiple general and specific safety practices (see section III) for each and every stage of the medication use process, aimed at reducing the incidence of errors associated with the use of medications included in its high-risk list. It is important to keep in mind that a single risk-reduction practice rarely suffices to prevent all possible incidents involving a high-risk medication.
The following recommendations should be taken into account in selecting safety practices for implementation:
- -
Safety practices should be implemented at every stage of the medication process (procurement, storage, prescribing, reviewing, dispensing, preparation, administration, monitoring, patient and caregiver education and care transitions) with engagement of all professionals involved.
- -
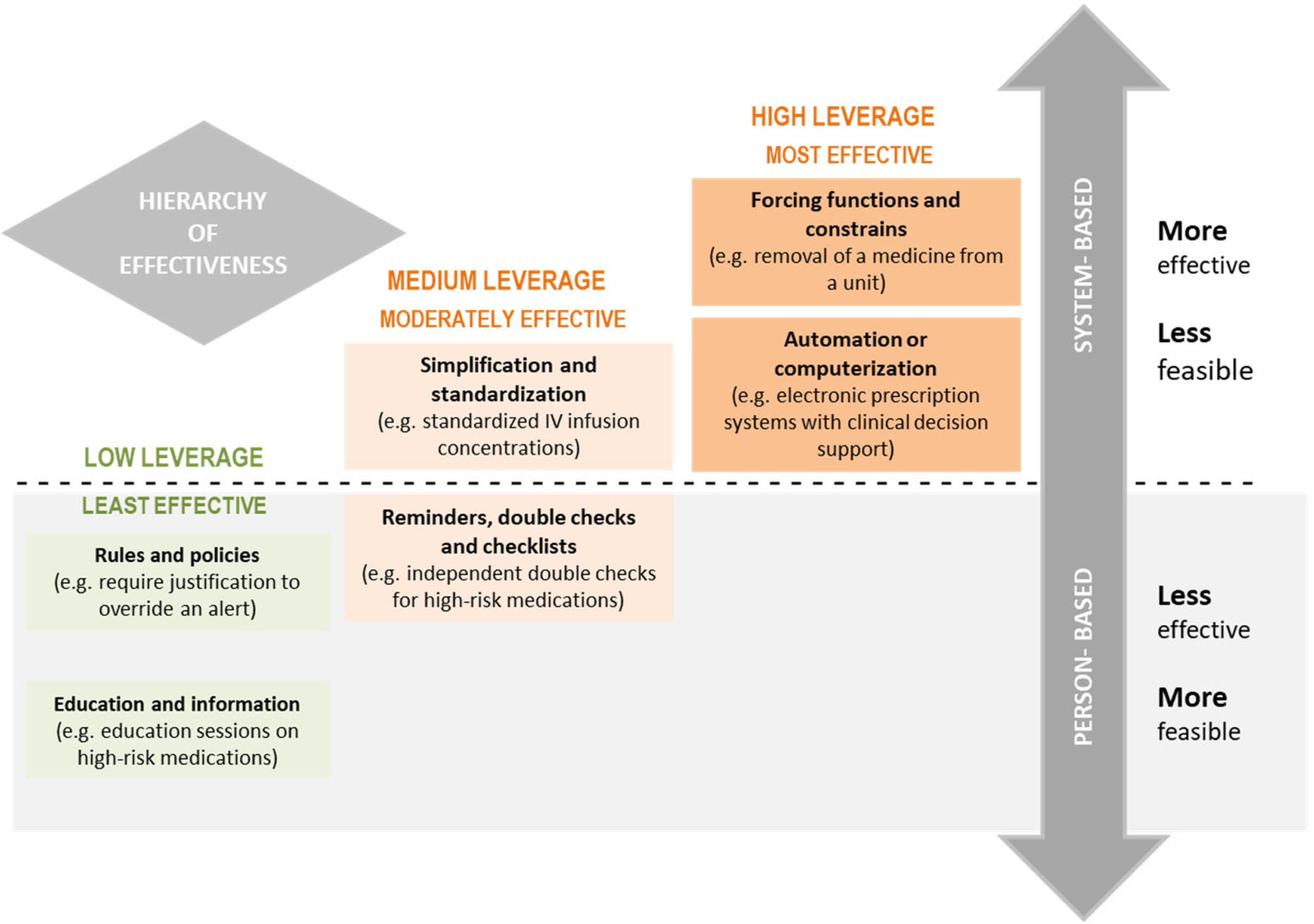
The “hierarchy of effectiveness” of error prevention practices should be taken into consideration (Fig. 1).26 It is recommended to prioritize highly effective practices, such as those involving automation and/or the use of technology, constraints or computerized alerts, or moderately effective ones, such as standardization and simplification. These should be combined with less effective practices requiring health care professionals to adhere to rules, protocols or procedures to avoid errors or those that may rely on memorization.26,27 It is important to note that improvement is more likely with the implementation of few but highly effective practices aimed at preventing the most frequent errors involving each high-risk medication compared to the implementation of a large number of less-effective practices.
Fig. 1.Hierarchy of effectiveness of medication error prevention practices26 (reproduced with the approval of ISMP-Canada).
The most effective practices are those involving systemic changes and that do not rely on memory, attention or human behavior, although their implementation is usually more complex. Medium-leverage practices reduce the probability of errors occurring. They are relatively quick and easy to implement, but they need constant reinforcement and updating. The least effective measures are those that are person-based. They are quicker and easier to implement, but they must be accompanied by other measures to achieve a safe system.
- -
Ensure that error prevention does not rely solely on low-effectiveness practices, such as labeling the drawers or boxes containing high-risk medications or disseminating the organization high-risk medication list or information on these medications.
- -
Limit the use of double-checking to specific critical points in the medication process and certain high-risk medications (e.g., chemotherapy or opioid infusions).
- -
Consider the root causes and factors that increase the likelihood of errors in pediatric patients. For instance, given that errors in pediatric patients frequently result from the need to perform calculations to adjust the dose based on age and/or body weight, or to manipulate commercial presentations by splitting or diluting due to the unavailability of pediatric formulations, specific safe practices aimed at detecting and reducing errors at these critical steps should be selected.
It is important to emphasize that each center must define and adapt these safety practices according to its available processes, workflows, and technologies, and that it must carry out strategic reflection and planning with a view to both the functional and structural evolution of the organization.
- 3
Include practices promoting active involvement of patients, family members and caregivers in the safe use of high-risk medications
Patients, parents and/or caregivers should be educated and informed about any prescribed high-risk medications and the possible errors and adverse effects that may occur. They should also be encouraged to take an active role in treatment and to ask any questions they may have about the medication. Special emphasis should be placed on the importance of proper storage to prevent accidental poisoning or misuse of these medications.
They should also be provided with written information and materials to help ensure safe use, in easily understandable language. To this end, it is recommended that original documents be used, preferably documents that are also available in electronic format and can not only be downloaded directly but also be integrated into electronic support, management, monitoring and educational resources. The recommendations document includes the information leaflet 10 Tips for Safely Administering Medication to Children, developed specifically for parents and family members.9
- 4
Disseminate the high-risk medication risk and established safety practices and train health care professionals
All professionals involved in the use of medicines should be informed of the list of high-risk medications and error-reduction practices. The following actions, among others, are recommended to this end:
- -
Designate specific staff to coordinate all the activities related to safety programs in pediatric care settings with the support and recognition of the management of the facility.
- -
Organize informational sessions or trainings to discuss the reasons for selecting the medications, medication errors and the harm to patients that could be prevented, as well as the importance of implementing each risk-reduction practice.
- -
Include a practical component in educational interventions, for instance, simulation-based learning, to train clinicians and care teams on the use of high-risk medications and early response to adverse drug events.
- -
Create incentives for reporting incidents involving high-risk medications, including medication errors and adverse drug reactions (for instance, by including them among the objectives and targets of the departments or units) so as to promote a safety culture among the health care staff.
- -
Promote multidisciplinary analysis of incidents occurring at the center and provide feedback on these incidents at regular intervals for learning purposes, including the measures adopted by the center.
- 5
Monitor the implementation of safety practices and evaluate their effectiveness
Health care organizations must establish medication process and outcome indicators to monitor the implementation and to evaluate the effectiveness of established safety practices. Evaluations should be conducted at regular intervals to measure and analyze outcomes with the Risk Management Committee, the Pharmacy and Therapeutics Committee, and the center’s management team.
In this regard, it is advisable to monitor the actions undertaken at the center through periodic analysis of reported incidents.
Finally, it should be noted that safety management in healthcare centers must be a dynamic process. Therefore, organizations should regularly review and update their list of high-risk medications and established safe practices, taking into account the causes of errors involving these medications at their own facility, their healthcare activity, and published strategies for improving the use of high-risk medications.
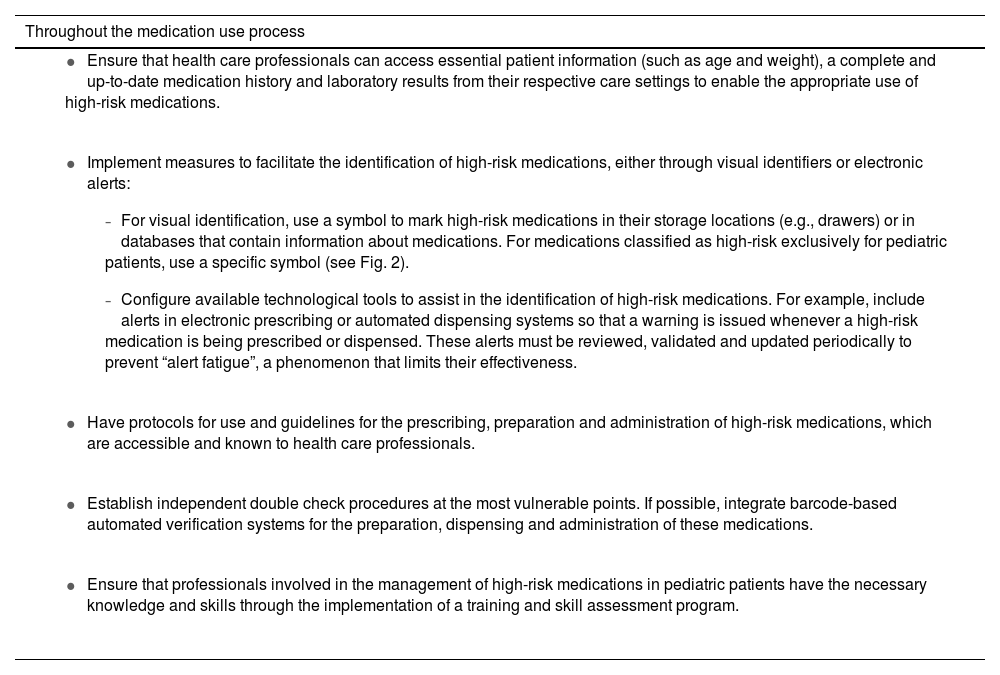
Section III. Safety practices to reduce errors in the use of high-risk medications in pediatric careThe document presents a series of general safety practices applicable to all high-risk medications (Table 2).3,4,8,20,22,28–39 These practices target each of the stages of the medication use process, from medication selection and procurement, through medication storage, prescribing, transcribing and reviewing, preparation, administration and monitoring to care transitions and patient and caregiver education. Although they focus on the safe use of high-risk medications in pediatric patients, many of them are also applicable to any type of medication or patient.
General safety practices to reduce errors involving high-risk medications in pediatrics.3,4,8,20,22,28–39
| Throughout the medication use process |
|---|
|
|
|
|
|
| Selection and procurement |
|---|
|
|
|
|
| Storage |
|---|
|
|
|
|
| Prescribing |
|---|
|
|
|
|
|
|
|
|
|
|
| Reviewing/dispensing |
|---|
|
|
| Preparation |
|---|
|
|
|
|
|
|
|
| Administration |
|---|
|
|
|
|
|
|
| Monitoring |
|---|
|
| Care transitions |
|---|
|
|
|
|
|
| Education of patients and caregivers |
|---|
|
|
|
|
|
|
In addition to these recommendations, the full version of the document describes the most frequent errors and specific safety practices for the following high-risk therapeutic classes or medications: IV adrenergic agonists, aminoglycosides, oral anticoagulants, heparin and other parenteral anticoagulants, neuromuscular blockers, insulin, medications for minimal and moderate sedation, parenteral nutrition, opioids, methotrexate (non-oncologic use), IV paracetamol, IV potassium and vancomycin. Due to length constraints, we were unable to summarize this information in the present article, so we recommend consulting the full version of the document.9
Health care centers should implement specific pediatric practices combined with general practices to improve safety, bearing in mind the criteria presented in Section II and the characteristics of the patients and care settings of the center. In pediatric care in particular, it is important to consider that certain subsets of patients, such as neonates, patients with complex chronic conditions, oncological patients and obese patients, and certain care settings, such as emergency departments, intensive care units, day hospitals, and hospital-at-home units, call for special considerations that are key to ensuring safe medication use.
In conclusion, safety in the use of high-risk medications in pediatric care is a crucial challenge that requires a comprehensive and multidisciplinary approach adapted to the specific characteristics of this vulnerable population. The development of programs for high-risk medication safety in health care organizations, with implementation of effective safety strategies at every stage of the medication process and promoting the education of health care professionals, patients and caregivers, is an essential step toward reducing medication errors and their adverse consequences. To succeed, this joint effort requires institutional commitment and ongoing evaluation of implemented interventions, thereby ensuring sustained improvement in pediatric patient safety and enhancing the safety culture of health care organizations.
Ethical considerationsThis article did not use any form of patient data.
FundingThis research did not receive any external funding.
The authors have no conflicts of interest to declare.
- •
Asociación Española de Pediatría:
- ◦
Committee on Health Care Quality and Patient Safety:
- -
Vanessa Arias Constanti (Hospital Sant Joan de Déu, Barcelona).
- -
Cristina Casado Reina (Primary Care Administration, Dirección Asistencial Norte de Madrid).
- -
Marta Duero Adrados (Hospital Sant Joan de Déu, Barcelona).
- -
Cristina Nebot Marzal (Centro de Salud de Campanar, Valencia).
- -
María José Salmerón Fernández (Hospital Universitario Virgen de las Nieves, Granada).
- -
Ángela Usarralde Pérez (Primary Care Administration, Dirección Asistencial Sur de Madrid).
- -
- ◦
Committee on Medicines:
- -
Raquel Escrig Fernández (Hospital Universitario La Fe, Valencia).
- -
Pedro Viaño Nogueira (Complejo Hospitalario Universitario de Pontevedra)
- -
- ◦
- •
Sociedad Española de Farmacia Hospitalaria, Grupo Español de Farmacia Pediátrica (Spanish Group of Pediatric Pharmacy):
- -
María José Cabañas Poy (Hospital Universitario Vall d’Hebron, Barcelona).
- -
Carmen Gallego Fernández (Hospital Regional Universitario de Málaga, Malaga).
- -
Isabel García López (Hospital Infantil Universitario Niño Jesús, Madrid).
- -
María Goretti López Ramos (Hospital Sant Joan de Déu, Barcelona).
- -
Margarita Ladrón de Guevara García (Hospital Universitario Virgen del Rocío, Seville).
- -
Cristina Martínez Roca (Complejo Hospitalario Universitario de A Coruña).
- -
Raquel Saldaña Soria (Hospital Regional Universitario de Málaga, Malaga)
- -
- •
Autonomous Communities:
- -
Andalusia: Elvira Eva Moreno Campoy, Margarita Ladrón de Guevara García, Paloma Trillo López, Elena Corpas Nogales and Estefanía López Domínguez.
- -
Aragon: Almudena Marco Ibáñez, Paloma Huerta Blas, Pilar Collado Hernández, María Teresa Llorente Cereza, Gloria Bueno Lozano, Sofía Valle Guillén, Olga Bueno Lozano and Gerardo Rodríguez Martínez.
- -
Asturias: Cristina Álvarez Asteinza, Iván Maray Mateos, Sonia Lareu Vidal, Andrés Concha Torre and Belén Suarez Mier.
- -
Canary Islands: Paloma García de Carlos, Nuria Bañón Morón and María José García Mérida.
- -
Cantabria: María Oro Fernández and José Luis Teja Barbero.
- -
Castilla y Leon: Tomás Maté Enríquez.
- -
Castilla-La Mancha: Sonia Cercenado Sorando, Marta García Palomo and Natalia Ramos Sánchez.
- -
Catalonia: Gloria Oliva Oliva, Laura Navarro Vila and Roser Bosser Giralt.
- -
Valencian Community: María José Merino Plaza.
- -
Galicia: Olga Roca Bergantiños.
- -
Madrid: Alberto Pardo Hernández, Maria José Calvo Alcántara and María de la Corte García.
- -
Murcia: José Eduardo Calle Urra, Amaya Jimeno Almazán, Teodoro José Martínez Arán and Mariana Tobaruela Soto.
- -
Navarre: Aránzazu Elizondo Sotro, Raquel Astiz Lizarraga, Regina Juanbeltz Zurbano and Leire Leache Alegría.
- -
Other members of the Spanish Association of Pediatrics, the Spanish Society of Hospital Pharmacy, and autonomous communities, which are presented in the Appendix, participated in the document.
This this article is being published simultaneously in Farmacia Hospitalaria (http://doi.org/10.1016/j.farma.2025.03.005) with the mutual consent of the authors and editors.