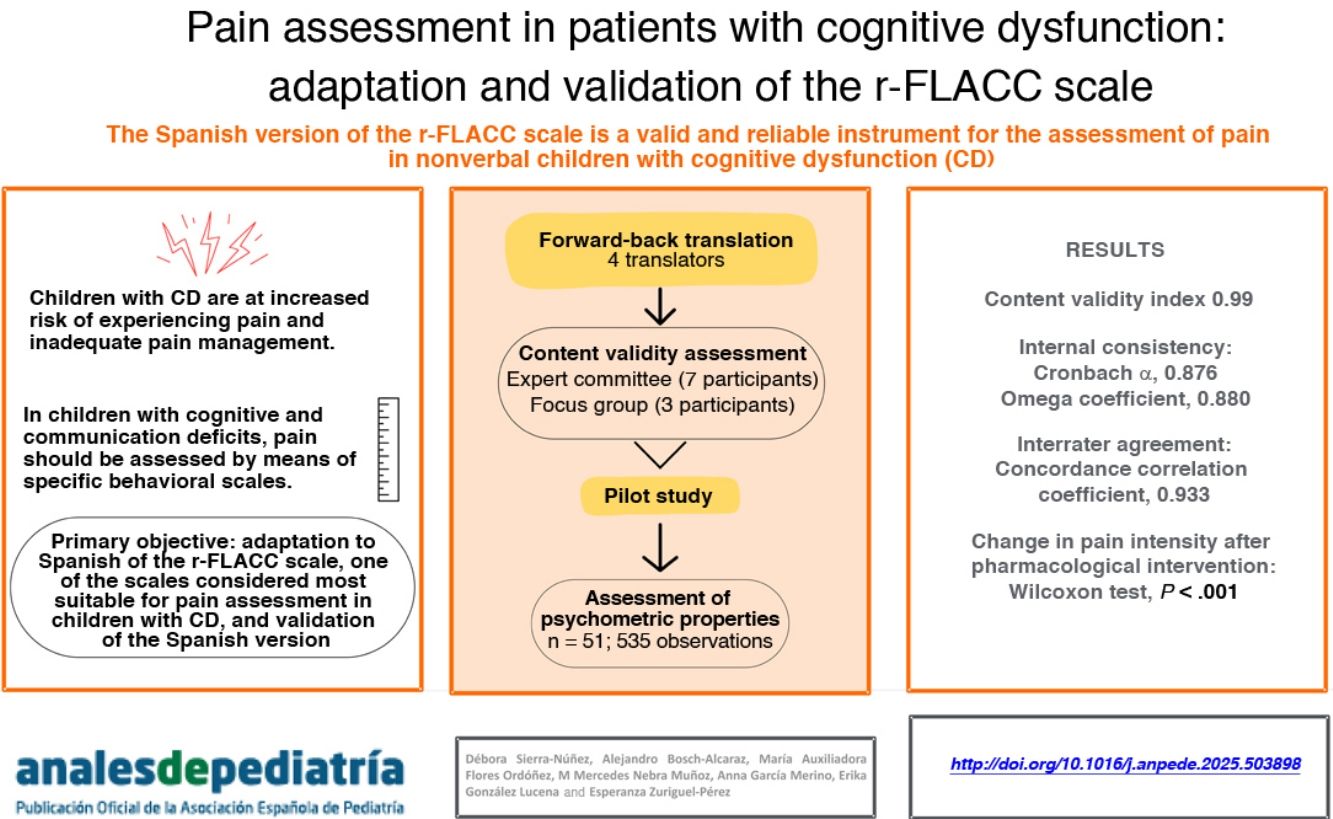
Minors with cognitive dysfunction are at increased risk for pain and inadequate pain management. Pain assessment in children with cognitive and verbal dysfunction should be performed using specific behavioral scales. The main objective of this study was the translation and adaptation to Spanish of the revised Face, Legs, Activity, Cry and Consolability (r-FLACC) scale, one of the best-rated scales for assessing pain in children with cognitive dysfunction, followed by its validation.
Patients and methodsWe carried out a prospective, observational and psychometric study in three phases: translation and adaptation, content validity analysis using mixed methods, and analysis of the psychometric properties of the Spanish version of the r-FLACC scale.
ResultsWe obtained an overall content validity index of 0.99, supported by qualitative data obtained in a focus group of patients’ relatives. The internal consistency of the Spanish version was demonstrated by a Cronbach α of 0.876 and a McDonald omega of 0.880; and the interobserver agreement by a concordance correlation coefficient of 0.933. The r-FLACC scale exhibited the capacity to detect changes in pain severity after pharmacological intervention, with P < 0.000 in the Wilcoxon test.
ConclusionsThe Spanish version of the r-FLACC scale proved to be a valid and reliable instrument for assessing pain intensity in children with cognitive dysfunction, thus providing professionals a tool that had not been available in our area and contributing to improving the scientific evidence available on the subject.
Los menores afectos de disfunción cognitiva presentan un riesgo mayor de sufrir dolor y de recibir un inadecuado manejo del mismo. La valoración del dolor en menores con disfunción cognitiva y verbal debe realizarse mediante el uso de escalas conductuales específicas. El objetivo principal de este estudio fue adaptar y validar al español la escala revisada Face, Legs, Activity, Cry and Consolability (r-FLACC), una de las escalas mejor valoradas para evaluar el dolor en menores con disfunción cognitiva.
Pacientes y métodosSe realizó un estudio observacional, psicométrico y prospectivo en tres fases: traducción y adaptación; análisis de validez de contenido mediante metodología mixta; y análisis de propiedades psicométricas de la escala r-FLACC versión española.
ResultadosSe obtuvo un índice de validez de contenido global de 0,99 apoyado por datos cualitativos generados en un grupo focal con familiares de pacientes. La consistencia interna fue demostrada con un alpha de Cronbach de 0,876 y un omega de McDonald de 0,880; y la concordancia interobservador con un coeficiente de correlación de concordancia de 0,933. La escala r-FLACC demostró capacidad para detectar cambios en el grado de dolor tras una intervención farmacológica, obteniendo una p < 0,000 en la prueba de Wilcoxon.
ConclusionesLa escala r-FLACC versión española resultó un instrumento válido y fiable para valorar la intensidad de dolor en menores con disfunción cognitiva, lo cual aporta a los profesionales una herramienta hasta ahora inexistente en nuestro contexto y contribuye a mejorar la evidencia científica disponible sobre el tema.
The factors that can trigger pain in children with cognitive dysfunction (CD) include, in addition to the usual triggers in children and adolescents, those arising from the symptoms associated with the underlying condition and increased exposure to surgery and other invasive medical procedures.1–4 As a result of this, which is compounded by the inability to verbally report the experience of pain, this population is at increased risk of inadequate pain management, which is directly related to the incorrect assessment of pain.3,5,6
Observational instruments capable of identifying this symptom based on specific behaviors are required for minors lacking the necessary cognitive or verbal skills to report it.2,5,7 Several behavioral scales have been developed for the assessment of pain based on the behaviors associated with pain commonly described in children with CD in the scientific literature.2,3,5,8–11 The revised Face, Legs, Activity, Cry and Consolability (r-FLACC) scale is one of the most appropriate behavioral scales to assess the intensity of pain in children with CD9 and is particularly useful for those who are nonverbal or with insufficient language skills to report the location and intensity of pain.8,9 In previous studies, professionals considered this instrument to be useful for clinical practice due to its ease of use and flexibility.2,3,5,8,12
Therefore, pain assessment in nonverbal children with CD poses a challenge to health care professionals. Some of the barriers hindering the correct assessment of pain are the lack of specific instruments, the lack of specific training and the scarcity of the evidence on pain assessment and management in children with CD.4,5,13–17 The r-FLACC scale has been adapted and validated in other populations and languages, such as Portuguese18 or Danish.19 However, when it comes to Spain, there is no valid and reliable instrument for assessing pain in children with CD, which was the reason for conducting this study. Our objective was to translate and adapt the r-FLACC scale and analyze the reliability and validity of the resulting Spanish version.
Patients and methodsDesignWe conducted an observational, psychometric and prospective study in 3 phases (translation to Spanish and back-translation to English, assessment of content validity and psychometric properties) between July 2022 and June 2024 in the pediatric surgery inpatient unit of a tertiary care hospital, in adherence to the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement20 and the Consensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN).21
Population and sampleThe translation and adaptation of the r-FLACC scale (phase 1) involved four health care professionals who were bilingual in Spanish and English with at least 5 years of clinical experience, including the management of pediatric patients with cognitive impairment. There were no exclusion criteria.
The assessment of content validity (phase 2) was performed with a mixed methodology that combined a sampling method that sought variation to maximize discursive diversity.22 A multidisciplinary group was formed, including seven experts23 selected the criteria recommended by Skjong and Wentworth (2001).24 In addition, we carried out a focus group with 3 family members of patients with CD. The selection criteria were being one of the main caregivers of a patient that had undergone surgery in the past two years and having been present during the postoperative hospital stay. We excluded caregivers with language barriers.
For the assessment of the psychometric properties of the Spanish version of the r-FLACC (phase 3), we selected pediatric patients according to the following criteria:
- -
Inclusion criteria:
- ◦
Age 2–18 years. Patients aged 2 years and older were included because this is the estimated age at which the main milestones of verbal communication are achieved (eg, production of 2–3 word sentences and use of pronouns). The r-FLACC scale was designed for use in nonverbal patients who have a disability or cognitive impairment that affects spoken language and, as we mentioned above, this type of impairment is difficult to diagnose before age 2 years.
- ◦
Diagnosis of a disease that manifests with CD.
- ◦
Surgical intervention within the past 72 h.
- ◦
Inability to verbally express the presence of pain.
- ◦
Admission to the study unit and provision of informed consent.
- ◦
- -
Exclusion criteria:
- ◦
Patients with caregivers who did not understand Spanish.
- ◦
Patients unaccompanied by family.
- ◦
Institutionalized patients.
- ◦
Patients at the end of life.
- ◦
- -
Withdrawal:
- ◦
Patients who required administration of sedative drugs or intravenous muscle relaxants in continuous infusion during the data collection process.
- ◦
Family’s express desire to withdraw from the study.
- ◦
We calculated the sample size following the recommendations of Norman and Streiner, which established a minimum of 10 participants per item for the assessment of validity and a minimum of 50 participants for the assessment of reliability.25
Study variablesDuring phase 3 of the study, we collected data on the following patient-related variables: (a) age; (b) sex; (c) nationality; (d) type of surgery; (e) previous surgeries; (f) principal diagnosis; (g) primary caregiver; (h) total length of stay in days; (i) pain intensity (assessed with the Spanish version of the r-FLACC).
Data collection instrumentsThe main instruments were the Spanish version of the r-FLACC scale and an ad hoc data collection form developed to record the sociodemographic and clinical characteristics of the patients.
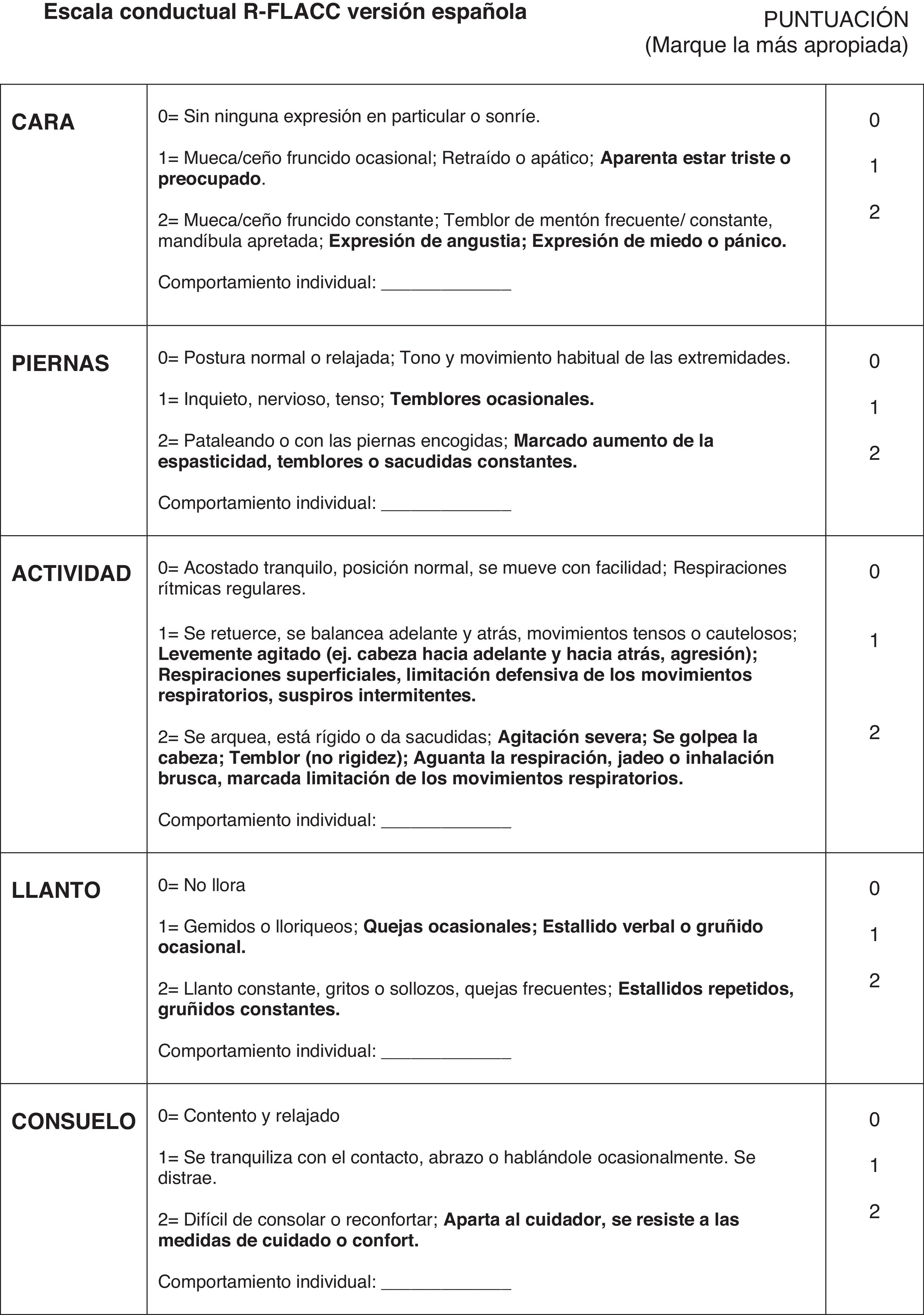
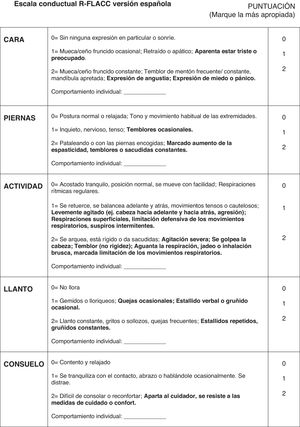
The r-FLACC scale, used to assess the intensity of pain in pediatric patients with CD, was developed as an adaptation of the Face, Legs, Activity, Cry and Consolability (FLACC) scale (1997).26 It incorporates additional specific descriptors for five categories of pain-related behavior: facial expression, leg movement, activity, cry and consolability. Each category is scored on a scale from 0 to 2 based on the observed behavior, yielding a total score between 0 (no pain) and 10 (worst possible pain) (Fig. 1).
The original r-FLACC scale exhibited a high interrater reliability with excellent intraclass correlation coefficients (ICC range, 0.76 to 0.90) and adequate κ statistics (0.44−0.57), and its construct validity was demonstrated by the decrease in pain scores following analgesic administration (mean, 6.1 [SD, 2.6] vs 1.9 [SD, 2.7]; P < .001).9
Data collection procedurePhase 1: Transcultural adaptationThe cross-cultural adaptation was based on the forward-and-back translation method, taking into account the recommendations proposed by the International Test Commission (ITC),27 followed by focus groups.
We sent the original r-FLACC scale in English to two professionals to translate it into Spanish, asking them to rate the difficulty of finding a conceptually equivalent expression for each item on a scale from 0 to 10. Once the Spanish version of the scale was produced, the two translators and the principal investigator met to analyze the translations, assess the difficulties encountered in the translation process for each item, resolve any identified discrepancies and generate the first version of the r-FLACC scale in Spanish.27,28 Then, another two translators independently produced the back translation to English, following the same protocol and holding the corresponding meeting to reach a consensus (Appendix A and B of supplemental material).
Following the quality-control checklist for adapted and translated items proposed by Hambleton and Zenisky (2010),29 the principal investigator and another member of the research team with experience in psychometric assessment conducted a qualitative analysis of the translations in order to: (a) assess the content, semantic, technical, criterion and conceptual equivalence of the forward translation to Spanish, back-translated version and original version of the r-FLACC scale (b) produce the final Spanish version of the scale and (c) evaluate its transcultural adaptation.
Phase 2: Content validity assessmentA quantitative assessment of the Spanish version of the r-FLACC was carried out with the collaboration of a multidisciplinary expert committee23 composed of seven professionals, who rated the clarity, coherence and relevance of the instrument on a Likert scale ranging from 1 (totally disagree) to 4 (totally agree) (Appendix C and D of supplemental material).
Subsequently, we conducted a qualitative assessment of the content validity of the Spanish version of the r-FLACC scale through a focus group, with participation of family members of patients with CD (Appendix E of supplemental material).30 We reached out to 22 family members in person or by e-mail to offer them to participate in the focus group, six of who accepted, but on the agreed-upon date, only 3 family members connected to the meeting.
The meeting, which lasted 1 h and 40 min, was held remotely through the Microsoft Teams account of the institution. The meeting started by informing participants of the purpose of the study and that the meeting would be recorded, requesting both their verbal and written consent.
Last of all, we carried out a pilot study, after which further modification of the instrument was not necessary, marking the end of the content validation phase of the Spanish version of the r-FLACC scale (Fig. 1).
Phase 3: Psychometric analysisIt was performed according to the recommendations of the COSMIN and the ITC guidelines.21,27
We recorded the pain intensity of each patient assessed with the Spanish version of the r-FLACC scale every 4 h in the first 48 h from admission. This assessment was made and recorded simultaneously and independently at the patient's bedside by the nurse assigned to the patient and another member of the research team. The clinician in charge of the patient was the one who determined the need to administer analgesia to the patient at each of the assessments. All patients who required analgesia underwent another pain assessment with the r-FLACC scale-Spanish version 30 min after its administration, following the same procedure.
Statistical analysisThe data were entered in the hospital’s REDCap platform and analyzed with the statistics package SPSS version 29 (IBM Corp; Armonk, NY, USA).
We summarized numerical data as mean and standard deviation or median and interquartile range and qualitative data as absolute frequencies and percentages.
For the quantitative assessment of content validity, we calculated item-level content-validity indices (I-CVIs) and the scale-level content validity index (S-CVI), following the recommendations of Polit and Tatano (2006). We considered values greater than 0.8 acceptable.31
We performed a confirmatory factor analysis and assessed reliability by means of the Cronbach alpha and the omega coefficient. Values between 0.8 and 0.9 were considered acceptable.
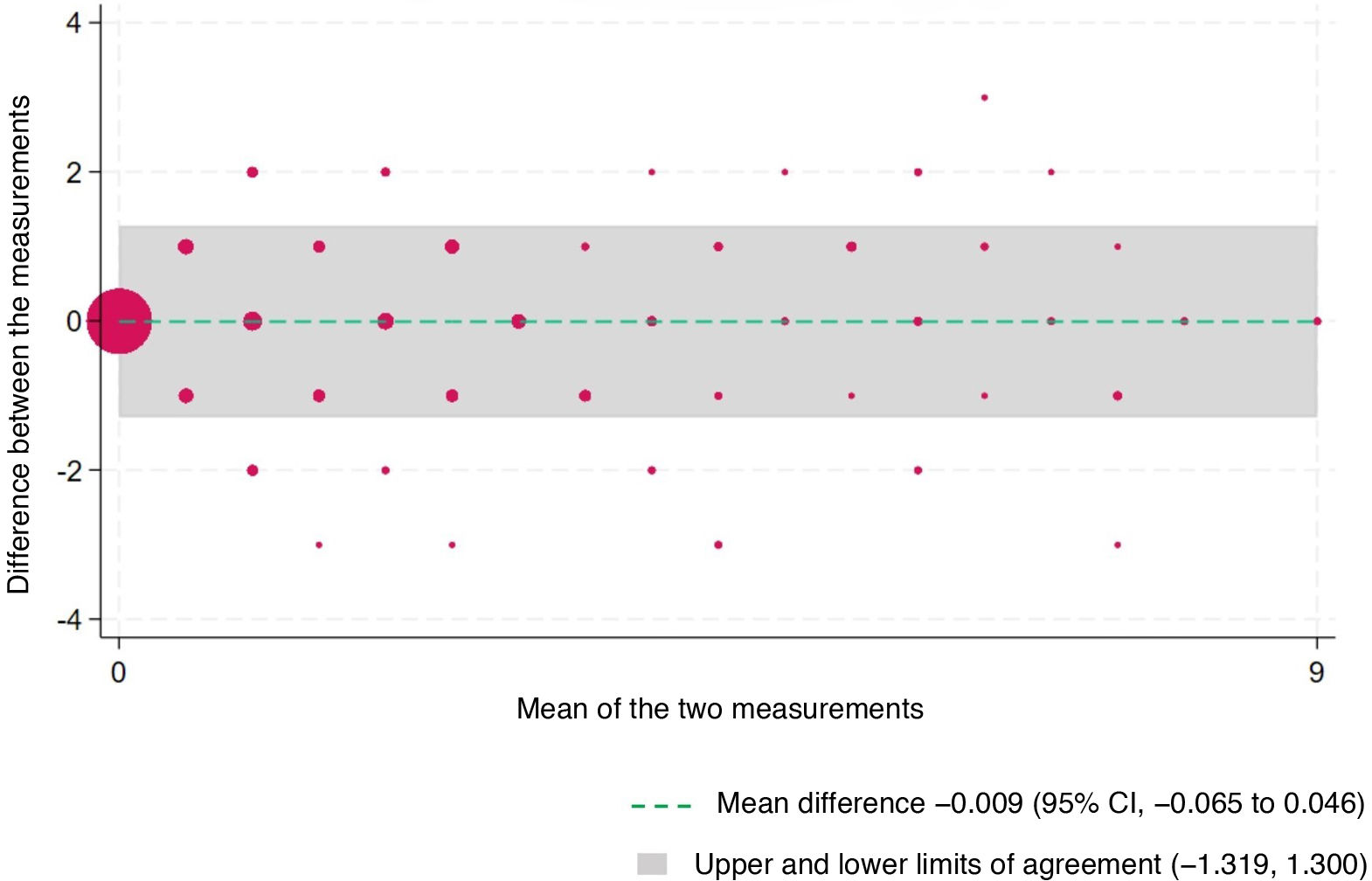
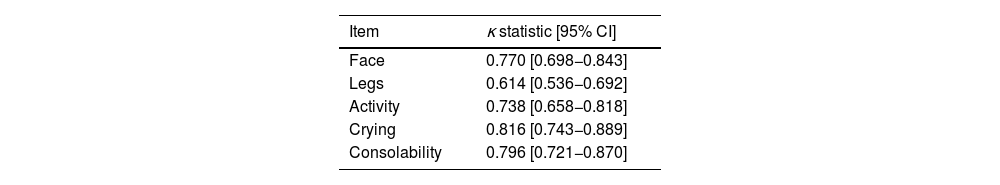
Interrater agreement was assessed by means of the Lin concordance correlation coefficient (rho_c), considering values near 1 good. We compared measurements graphically by means of Bland-Altman plots and calculated κ correlation coefficients for each item, applying the following thresholds: 0.00, no agreement; 0.01 to 0.20, slight; 0.21 to 0.40, fair; 0.41 to 0.60, moderate; 0.61 to 0.80, substantial; 0.81–1.00, near perfect.
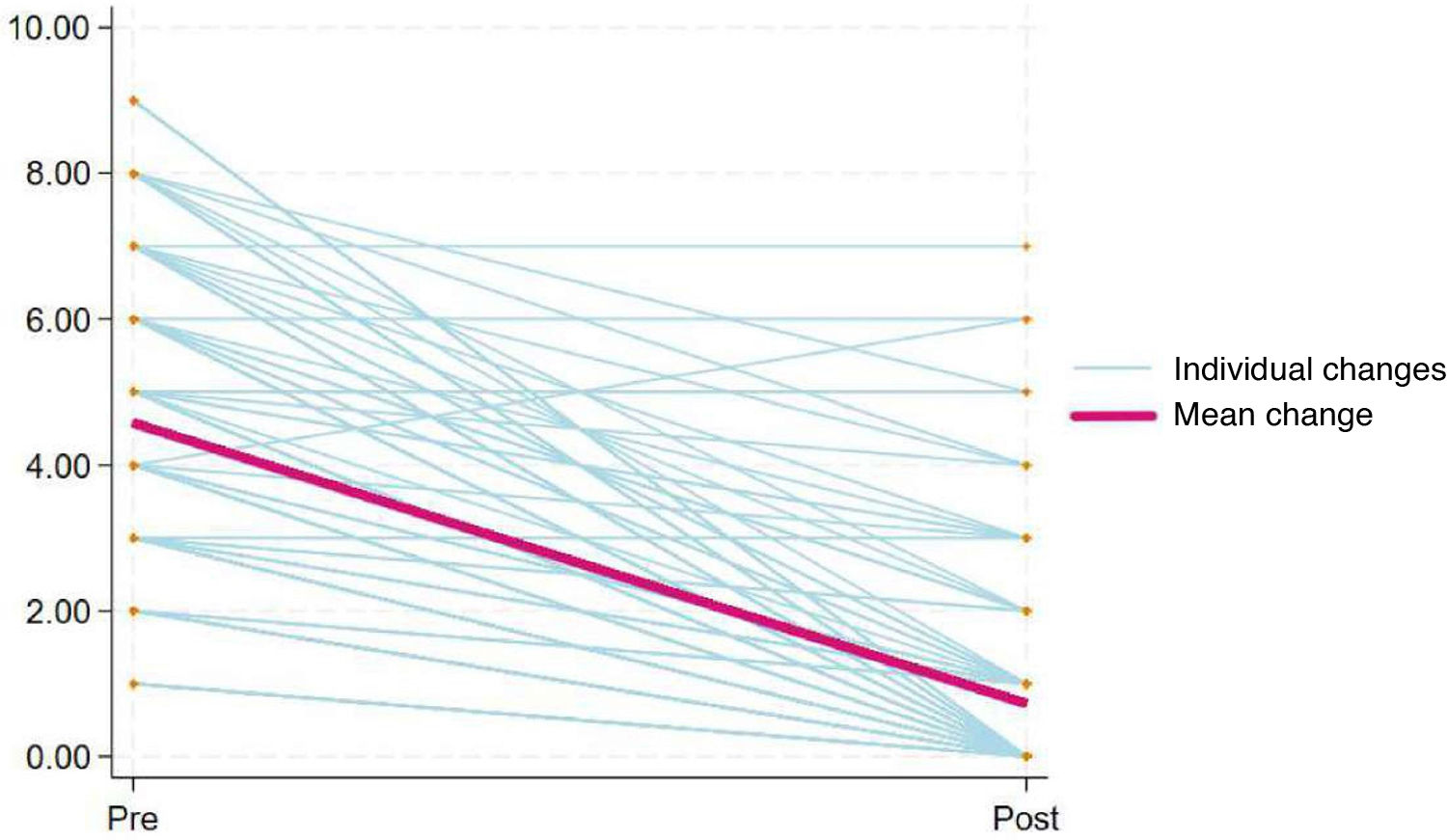
To determine the sensitivity to change of the scale, we assessed the differences between pre- and post-analgesia scores and used the Wilcoxon signed-rank test. We considered P values of less than 0.05 statistically significant for all tests.
Ethical considerationsThe study was approved by the research ethics committee of the hospital center and we obtained the license for the use of the original r-FLACC scale through the University of Michigan. The study was conducted in adherence to the principles of the Declaration of Helsinki (2013) and the Belmont Report (1979). Participants were informed about the study verbally and in writing, and we obtained informed consent to participation. The use and handling of data adhered to Regulation 2016/679 of the European Parliament and Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data and Organic Law 3/2018 of 5 December on the protection of personal data and the guarantee of digital rights.
ResultsContent validity and patient characteristicsA total of seven professionals participated in the quantitative content validity analysis. We obtained I-CVIs ranging from 0.8 to 1 and a S-CVI of 0.99.
All family members that participated in the focus group agreed that the r-FLACC scale was a useful tool for assessing the intensity of pain in a pediatric patient with CD who is unable to report it verbally.
No problems arose in the pilot study, so we did not modify the scale prior to phase 3.
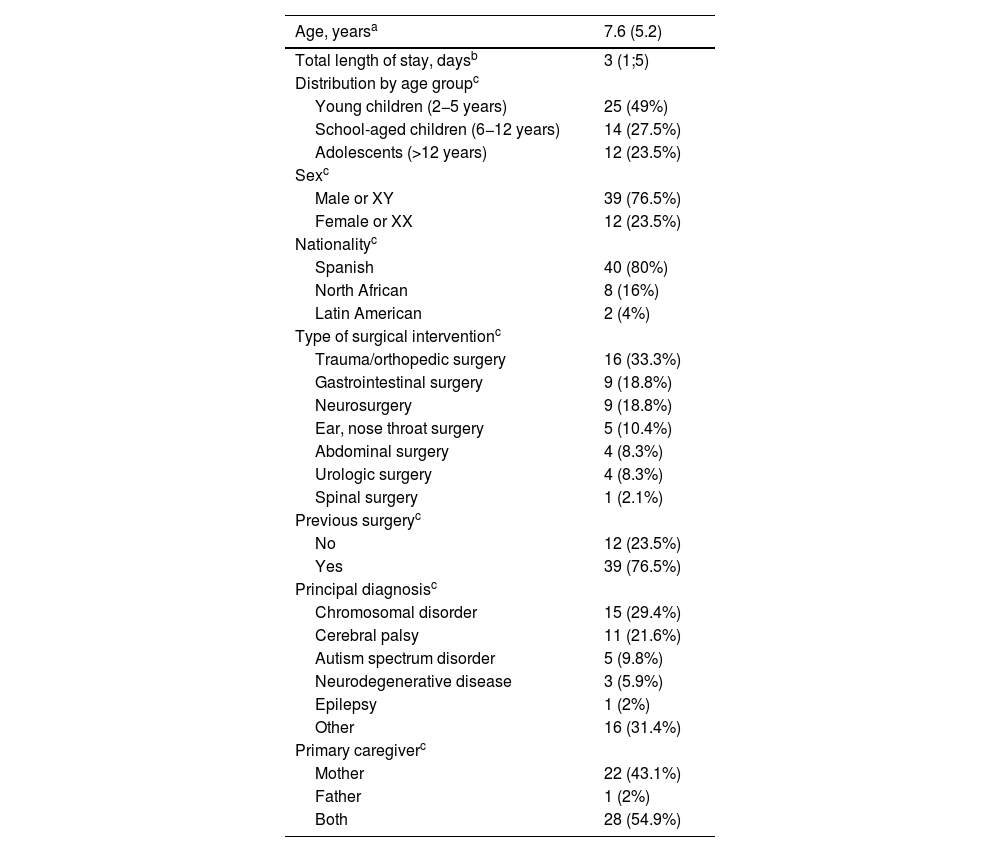
In phase 3, there were 52 patients in the initial sample, but only 51 were finally included because the remaining one required initiation of intravenous sedation by continuous infusion. A total of 535 simultaneous observations were performed, amounting to a total of 1073 assessments made with the Spanish version of the r-FLACC. In this phase, 76.5% of the patients (n = 39) were male, and the mean age was 7.6 years (SD, 5.2) (Table 1).
Sociodemographic and clinical characteristics of the patients.
| Age, yearsa | 7.6 (5.2) |
|---|---|
| Total length of stay, daysb | 3 (1;5) |
| Distribution by age groupc | |
| Young children (2−5 years) | 25 (49%) |
| School-aged children (6−12 years) | 14 (27.5%) |
| Adolescents (>12 years) | 12 (23.5%) |
| Sexc | |
| Male or XY | 39 (76.5%) |
| Female or XX | 12 (23.5%) |
| Nationalityc | |
| Spanish | 40 (80%) |
| North African | 8 (16%) |
| Latin American | 2 (4%) |
| Type of surgical interventionc | |
| Trauma/orthopedic surgery | 16 (33.3%) |
| Gastrointestinal surgery | 9 (18.8%) |
| Neurosurgery | 9 (18.8%) |
| Ear, nose throat surgery | 5 (10.4%) |
| Abdominal surgery | 4 (8.3%) |
| Urologic surgery | 4 (8.3%) |
| Spinal surgery | 1 (2.1%) |
| Previous surgeryc | |
| No | 12 (23.5%) |
| Yes | 39 (76.5%) |
| Principal diagnosisc | |
| Chromosomal disorder | 15 (29.4%) |
| Cerebral palsy | 11 (21.6%) |
| Autism spectrum disorder | 5 (9.8%) |
| Neurodegenerative disease | 3 (5.9%) |
| Epilepsy | 1 (2%) |
| Other | 16 (31.4%) |
| Primary caregiverc | |
| Mother | 22 (43.1%) |
| Father | 1 (2%) |
| Both | 28 (54.9%) |
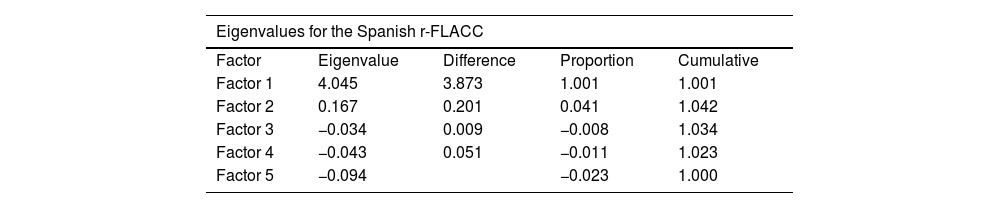
We performed a principal components analysis and found that the Spanish version of the r-FLACC scale consists of a single component with 5 items (Table 2).
Confirmatory factor analysis of the Spanish version of the r-FLACC scale.
| Eigenvalues for the Spanish r-FLACC | ||||
|---|---|---|---|---|
| Factor | Eigenvalue | Difference | Proportion | Cumulative |
| Factor 1 | 4.045 | 3.873 | 1.001 | 1.001 |
| Factor 2 | 0.167 | 0.201 | 0.041 | 1.042 |
| Factor 3 | −0.034 | 0.009 | −0.008 | 1.034 |
| Factor 4 | −0.043 | 0.051 | −0.011 | 1.023 |
| Factor 5 | −0.094 | −0.023 | 1.000 | |
| Item-total correlation for the Spanish version of the r-FLACC scale | |||
|---|---|---|---|
| Item | Factor 1 | Factor 2 | Unit |
| Face | 0,933 | −0,093 | 0,120 |
| Legs | 0,804 | 0,253 | 0,290 |
| Activity | 0,883 | 0,207 | 0,177 |
| Crying | 0,937 | −0,151 | 0,100 |
| Consolability | 0,931 | −0,169 | 0,105 |
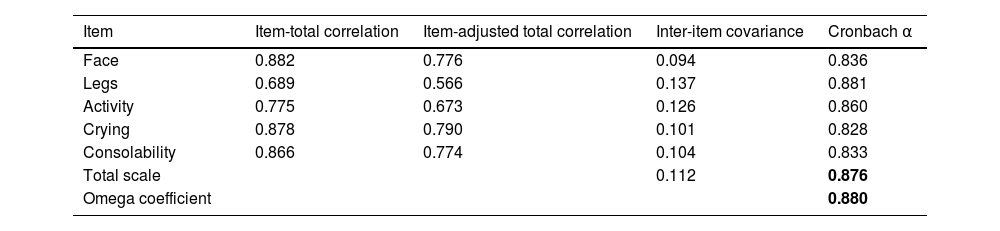
The Cronbach alpha for the overall scale was 0.876, with values ranging between 0.828 and 0.881 for each of the items. The omega coefficient was 0.880 (Table 3).
Internal consistency of the Spanish version of the r-FLACC.
| Item | Item-total correlation | Item-adjusted total correlation | Inter-item covariance | Cronbach α |
|---|---|---|---|---|
| Face | 0.882 | 0.776 | 0.094 | 0.836 |
| Legs | 0.689 | 0.566 | 0.137 | 0.881 |
| Activity | 0.775 | 0.673 | 0.126 | 0.860 |
| Crying | 0.878 | 0.790 | 0.101 | 0.828 |
| Consolability | 0.866 | 0.774 | 0.104 | 0.833 |
| Total scale | 0.112 | 0.876 | ||
| Omega coefficient | 0.880 |
The overall internal consistency for the Spanish version of the r-FLACC scale is presented in boldface.
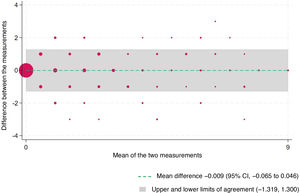
The concordance correlation coefficient (rho_c) was 0.933 (95% CI, 0.922−0.944) and statistically significant (P = .000). The quotient between the concordance correlation coefficient and the Pearson correlation coefficient was 1.000. Fig. 2 presents the Bland-Altman analysis of the agreement between rater A and rater B (n = 535).
Lastly, the obtained κ coefficients ranged between 0.614 and 0,816 (Table 4).
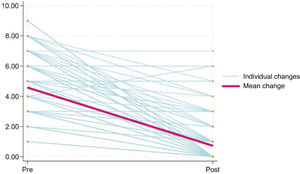
Sensitivity to changeWe assessed the sensitivity to change by comparing pain intensity scores before and after administration of analgesia in 130 observations (Fig. 3), obtaining a P value of less than .001 in the Wilcoxon signed-rank test, which indicates that the Spanish version of the r-FLACC is capable of detecting changes in pain intensity in pediatric patients with CD following pharmacological intervention.
DiscussionBased on the current literature, health care professionals consider the r-FLACC scale a useful instrument for assessing pain in children with CD due to its flexibility and ease of use,2,3,5,8,12 which was corroborated by the experts and family members who participated in our study. In addition, our analysis demonstrated adequate validity, reliability and sensitivity to change, which suggests that the Spanish version of the r-FLACC scale can have a significant impact in the management of pain in pediatric patients with CD
We carried out the transcultural adaptation of the r-FLACC scale following the recommendations of the International Test Commission (ITC). As Bussotti et al. did before us in the cultural adaptation of the scale to Brazilian Portuguese,18 we chose the forward and back translation method. However, we used a combined methodological approach for the assessment of content validity, adding a qualitative analysis that reflected the perspective of families to the conventional quantitative analysis by experts. Most of the instruments used for pain assessment in pediatric patients with CD have been developed with information provided by families about their children's pain behaviors,9–11 which motivated us to seek their participation.
We assessed the psychometric properties of the Spanish version of the r-FLACC scale in a sample of 51 patients, which was very similar to that of the original study.9 However, our analysis included a larger number of observations compared to the study conducted by Malviya et al9 (535 compared to 80).
It is worth noting that our study was conducted to adapt the r-FLACC scale to the Spanish population to be applicable in any pediatric patient incapable of verbally expressing their pain, in contrast to the study of Pedersen et al.,19 in which the sample (n = 27) consisted exclusively of children with cerebral palsy, or the study by Malviya et al.,9 in which the sample of children with CD included some who were able to communicate their pain verbally.
The assessment of the Spanish version of the r-FLACC scale yielded a Cronbach α of 0.876 for the total scale and α values ranging from 0.828 to 0.881 for individual items, which are acceptable, but slightly lower compared to the study by Pedersen et al.19 (0.902−0.975). Still, the values obtained in our study were similar to the values obtained in other studies of instruments used to assess pain in children with CD, such as the Paediatric Pain Profile (PPP, 0.75) or the Non-Communicating Children's Pain Checklist-Postoperative Version (NCCPP-PV, 0.91). Another difference in relation to the studies conducted by Malviya et al.9 and Pedersen et al.19 is that we also calculated the omega coefficient, which is currently considered a more accurate and reliable alternative to the Cronbach α for the assessment of reliability in the psychometric evaluation of scales.32 In addition, we found a good interrater agreement based on the Lin concordance correlation coefficient and the κ coefficients for individual items reflected substantial agreement, with the exception of the coefficient for the Legs item, which reflected near-perfect agreement. The concordance correlation coefficient of 0.933 was consistent with the intraclass correlation coefficient reported by Malviya et al.9 (0.90) and reflected slightly greater agreement compared to the value reported by Pedersen et al.19 (0.745).
In line with the study by Malviya et al.,9 the reduction in pain scores after administration of analgesia supports the construct validity of the Spanish version of the r-FLACC, as it demonstrates that the instrument measures the construct for which it was designed, which is pain.
One of the potential limitations of this study is that only three family members participated in the qualitative content validity analysis. Although the focus group offered added value in combination with the conventional content analysis, a focus group with more family members could have generated greater discursive diversity. A possible limitation of this study is that only three family members participated in the qualitative content validity analysis. Although this added value to the conventional content analysis, a focus group with more family members could have offered greater discursive diversity. Another potential limitation is that, in contrast to the studies conducted by Malviya et al.9 and Pedersen et al.,19 we did not assess criterion validity because a valid and reliable instrument was not available in Spain to assess pain in children with cognitive and communication deficits that could serve as the gold standard.
In conclusion, the Spanish version of the r-FLACC scale yielded very good psychometric results in terms of validity, reliability and sensitivity to change, contributing to improving the scientific evidence on pain assessment in children with CD. Our study demonstrated the reproducibility of pain assessments performed with the Spanish version of the r-FLACC in children from age 2 years, but we were unable to ascertain whether this was also the case in patients with CD aged 1 month to 2 years. In this age group, the use of preverbal behavioral scales is recommended. Children with CD are at increased risk of experiencing pain and of inadequate pain assessment due to the lack of a specific assessment tool.1–6 Therefore, the introduction of the Spanish version of the r-FLACC scale, which was previously unavailable, may contribute to remedying this lack for the professionals that manage these patients.
FundingThis study was funded by the Fundación Enfermería and Sociedad del Colegio Oficial de Enfermeros y Enfermeras of Barcelona in the framework of the grant program for research in nursing (grant PR-536/2022).
The authors have no conflicts of interest to declare.