Problems related to prematurity are the main cause of morbidity in newborns and infants. Between 80% and 85% of preterm babies are born between 320 and 366 weeks of gestation. In Spain, there is no guide for the perinatal management and long-term follow-up of moderately and late preterm (MLPT) infants.
For this reason, the SEN32-36 group of the Spanish Society of Neonatology has developed a guideline with recommendations for both perinatal management (prenatal care, initial care in the delivery room and ward, neonatal respiratory pathology, breastfeeding and feeding difficulties, hyperbilirubinaemia and hospital discharge) and long-term follow-up (neurodevelopment, nutrition and follow-up, prevention of respiratory disease after discharge and immunizations), grading them according to the Agency for Health Care Research and Quality scales of research quality and levels of evidence. The main goal was improving and standardizing the care of MLPT infants.
Los problemas relacionados con la prematuridad representan la principal causa de morbimortalidad neonatal e infantil. El 80-85% de los prematuros nacen entre las 320 y 366 semanas de gestación. En España, no se dispone de una guía para el manejo perinatal y el seguimiento a largo plazo de los prematuros moderados y tardíos (PMT).
Por este motivo, el grupo SEN32-36 de la Sociedad Española de Neonatología, ha elaborado una guía de recomendaciones tanto en el manejo perinatal (asistencia prenatal, asistencia inicial en sala de partos y en la planta de hospitalización, enfermedad respiratoria neonatal, lactancia materna y dificultades de alimentación, hiperbilirrubinemia y alta hospitalaria), y sobre el seguimiento a largo plazo (neurodesarrollo, nutrición y seguimiento, prevención de enfermedad respiratoria tras el alta e inmunizaciones) clasificándolas según la escala de gradación de la calidad de la evidencia científica de Agency for Health care Research and Quality. Con el objetivo principal de mejorar y homogenizar los cuidados de los PMT.
Problems related to prematurity are the leading cause of neonatal and pediatric morbidity and mortality. In Spain, the preterm birth rate is of approximately 7%,1 and of all preterm births, 80%–85% are moderate to late preterm (32+0 a 36+6 weeks’ gestation). If we consider the health care burden attributable to prematurity, moderate and late preterm (MLPT) birth is associated with a longer median length of stay (7 additional days), a higher frequency of visits to specialists after discharge (3.1-fold increase) and greater health care expenditures through age 5 years (1.2-fold increase) compared to full-term (FT) birth,2 in spite of which the risks and health care needs associated with MLPT birth continue to be underestimated.
The SEN32-36 working group of the Sociedad Española de Neonatología (Spanish Society of Neonatology) focuses on infants born preterm between 32 and 36 weeks’ gestation. A survey conducted in 2021 evinced substantial heterogeneity in the perinatal management of this subset of patients among Spanish hospitals.3 This motivated the performance of a systematic review on the perinatal management and follow-up of these infants in order to develop a clinical practice guideline and standardize the care of MLPT newborns.
MethodsLiterature search in the PubMed/MEDLINE, Cochrane Library, UpToDate and Google Scholar databases, limited to sources published between 2008 and February 2024, selecting the most recent articles that fit the search parameters (Appendix B, Supplemental material, Fig. S1) for the development of the guideline. The quality of the evidence and the grading of the recommendations were classified according to the scales developed by the Agency for Healthcare Research and Quality (AHRQ) (Appendix B, Supplemental material, Tables S1 and S2).
Following the critical appraisal of the evidence, the group proceeded to the development of recommendations grouped based on the different aspects under consideration from the perinatal period to long-term follow-up. Each of the seven components of the SEN32-36 group developed part of the recommendations, following a formal assessment model over 6 meetings held online to achieve complete consensus among the members of the group. In addition to the volume and quality of the evidence, the group considered its consistency and relevance in terms of its applicability to the Spanish health care system. In the case of clinical questions for which the evidence was scant, of poor methodological quality or inconsistent, the group produced consensus-based recommendations.
Prenatal careObstetricians must assess the risk of preterm birth and anticipate the need for treatment. When it comes to the administration of antenatal steroids, most countries have adopted the indication for anticipated preterm delivery up to 34 weeks’ gestation,4,5 although the American College of Obstetricians and Gynecologists (ACOG) maintains the indication through 36+6 weeks. There is evidence that steroid administration very close to birth promotes the development of hypoglycemia in the newborn6 and is associated with an increased risk of childhood mental disorders in the long term (adjusted hazard ratio [aHR], 1.13–1.33),7 although these unfavorable outcomes were not observed at 6 years in the Antenatal Late Preterm Steroids (ALPS) follow-up study.8
Recommendations- -
Administer prenatal corticosteroids in the case of anticipated preterm birth up to 34 weeks’ gestation (Grade A recommendation. Level of evidence: Ib). Each center should individualize administration of prenatal corticosteroids up to 34+6 weeks.4,5
- -
Scheduling a prenatal visit with the parents is recommended (Grade C recommendation).
After birth, neonatal morbidity is significantly greater compared to FT infants and increases with decreasing gestational age, and these diseases tend to be of mild to moderate severity. Neonatal morbidity is associated with a more complicated transition to extrauterine life and increased risk of hypothermia, hypoglycemia, respiratory distress, difficulty feeding, jaundice and infection.9
RecommendationsSee Table 1.
Initial management of moderate to late preterm neonates in the delivery room and maternity/neonatal ward.
| Delivery room |
| - Do not have the mother give birth alone, unaccompanied by her support person, unless this is her explicit wish, even in the case of cesarean delivery10 (Grade C recommendation) |
| - Whenever possible, delay cord clamping for at least 60 s11 (Grade A recommendation. Level of evidence: Ib) |
| - Initiate skin-to-skin contact immediately.12 Delay administration of vitamin K and eye prophylaxis, ensuring its subsequent administration either in the delivery room or the ward, according to the protocol of each hospital (Grade A recommendation. Level of evidence: Ib) |
| - If the newborn needs support to transition to extrauterine life, follow the recommendations published by the Group on Neonatal Resuscitation of the SENeo. (Grade A recommendation. Level of evidence: Ib) |
| - Confirm gestational age: ideally, combining the date of the last menstruation and the findings of the first trimester ultrasound, or based on the clinical assessment (Dubowitz/Ballard examination for gestational age). (Grade C recommendation) |
| Maternity/neonatal ward (Grade C recommendation) |
| - Periodic body temperature measurement in the first 24 h post birth |
| - Defer head washing or tub bathing of newborn until adequate thermal stability has been achieved |
| - Frequent assessments of clinical condition (heart rate, tissue perfusion, work of breathing, muscle tone…): every 30 min in the first 2 h and every 2–4 h thereafter through 24 h post birth |
| - Assessment of perinatal factors that increase the risk of respiratory problems |
| - Neonates born before 35 weeks’ gestation should receive special care due to the increased risk of perinatal disease. Each hospital should develop a protocol outlining the monitoring of the clinical condition of these MLPT infants according to its human and technological resources and physical infrastructure |
| - Avoid separating the mother and the infant, as long as the clinical condition of both allows it |
| - Establish a care delivery model that allows rooming-in for the mother and infant, which should not hinder in any way the performance of necessary assessments or delivery of the care needed by the infant |
| - Early treatment of any clinical abnormality that requires transfer of the neonate to the neonatal unit for specific treatment |
| - Allow access to the neonatal unit of parents and family members/accompanying persons at all times and implement a family-centered and development-focused care model |
Abbreviations: MLPT, moderate to late preterm; SENeo: Sociedad Española de Neonatología.
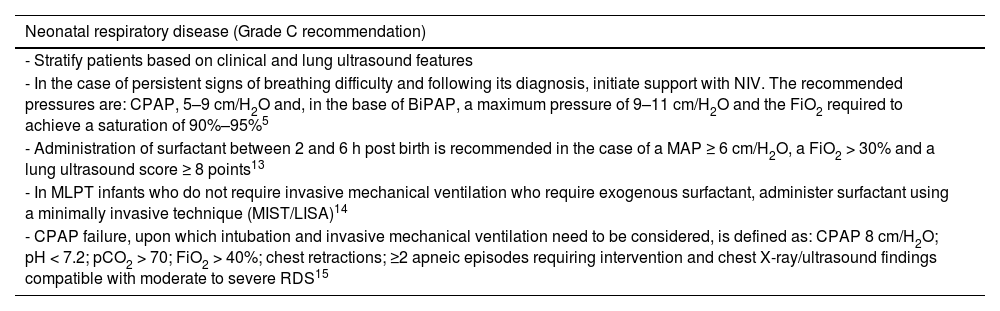
The most frequent respiratory disease in MLPT infants is transient tachypnea of the newborn, followed by respiratory distress syndrome (RDS). The usual treatments in this group of newborns are non-invasive ventilation (NIV) and, in a lesser percentage, exogenous surfactant administration. The definitive diagnosis must be made based on the clinical manifestations and imaging findings, radiography or preferably a thoracic ultrasound, which allows assessment of respiratory/lung patterns, classification of the patient based on the lung ultrasound score and delivery of appropriate treatment.
RecommendationsSee Table 2.
Management of neonatal respiratory disease in moderate to late preterm neonates.
| Neonatal respiratory disease (Grade C recommendation) |
|---|
| - Stratify patients based on clinical and lung ultrasound features |
| - In the case of persistent signs of breathing difficulty and following its diagnosis, initiate support with NIV. The recommended pressures are: CPAP, 5–9 cm/H2O and, in the base of BiPAP, a maximum pressure of 9–11 cm/H2O and the FiO2 required to achieve a saturation of 90%–95%5 |
| - Administration of surfactant between 2 and 6 h post birth is recommended in the case of a MAP ≥ 6 cm/H2O, a FiO2 > 30% and a lung ultrasound score ≥ 8 points13 |
| - In MLPT infants who do not require invasive mechanical ventilation who require exogenous surfactant, administer surfactant using a minimally invasive technique (MIST/LISA)14 |
| - CPAP failure, upon which intubation and invasive mechanical ventilation need to be considered, is defined as: CPAP 8 cm/H2O; pH < 7.2; pCO2 > 70; FiO2 > 40%; chest retractions; ≥2 apneic episodes requiring intervention and chest X-ray/ultrasound findings compatible with moderate to severe RDS15 |
Abbreviations: BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen; MAP, mean airway pressure; MIST/LISA, minimal invasive surfactant therapy/less invasive surfactant administration; MLPT, moderate to late preterm; NIV, noninvasive ventilation; pCO2, partial pressure of carbon dioxide; RDS, respiratory distress syndrome.
Moderate to late preterm newborns are at increased risk of hypoglycemia because glycogen and fat stores accumulate in the fetus starting in the third trimester. After birth, the maintenance of glucose homeostasis depends on the activation of various endocrine and metabolic systems that are impaired in preterm infants. The delay in the enzymatic activity of glucose-6-phosphatase, which hinders both glycogenolysis and gluconeogenesis, decreased nutritional intake due to gastrointestinal immaturity and dysfunctional sucking contribute to the risk of hypoglycemia.16 For these reason, infants born between 32 and 34 weeks’ gestation receive supplementation from birth and late preterm infants born at or after 35 weeks are included in most hypoglycemia management protocols for FT infants.
In recent years, some hypoglycemia management protocols have introduced the administration of oral 40% dextrose gel combined with feeding in infants in whom hypoglycemia does not resolve after feeding.17
Recommendations- -
Identify perinatal risk factors in the context of moderate to late preterm birth for the development of hypoglycemia: (Grade C recommendation)
- •
Maternal: hypertension, diabetes, obesity, administration of tocolytic agents, administration of intravenous dextrose before and during delivery, prolonged or complicated delivery.
- •
Fetal and neonatal: non-reassuring fetal status, intrauterine growth restriction, multiple gestation, 5-min Apgar score <7, hypothermia or thermal instability, sepsis, respiratory distress, polycythemia/hyperviscosity.
- •
- -
Optimize feeds. (Grade B recommendation. Level of evidence: III)
- -
The blood glucose target varies in the first 48 h of life, although a level greater than 50 mg/dL should be maintained from 24 h and a level greater than 60 mg/dL from 48 h. (Grade C recommendation)
- -
Hyperglycemia management protocol.18 (Appendix B, Supplemental material, Fig. S2)
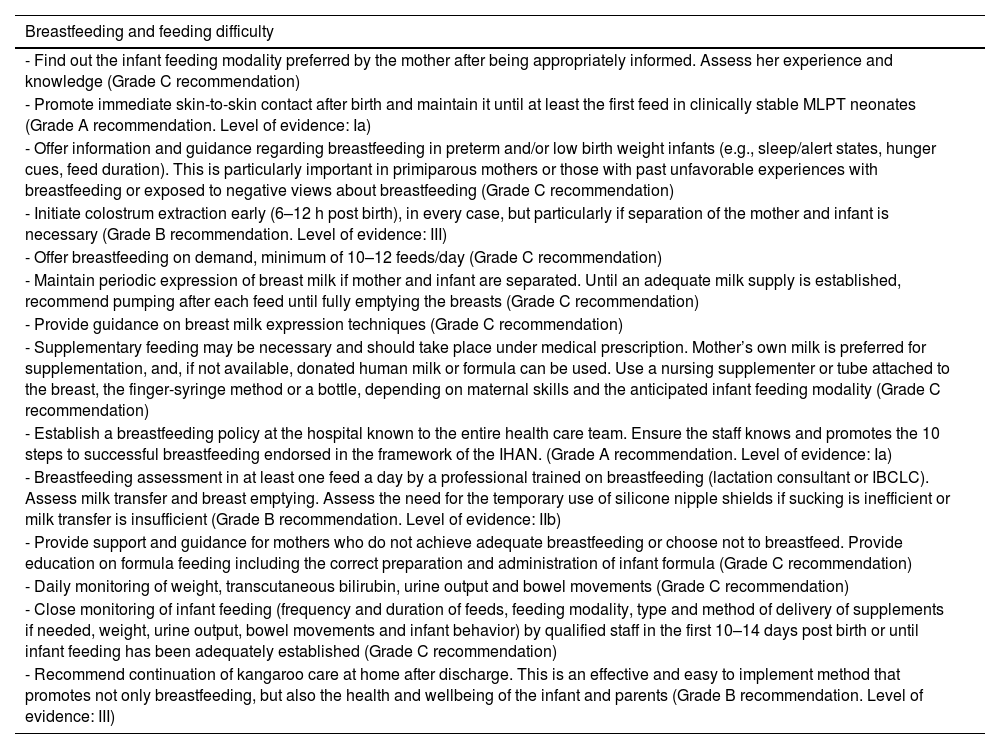
Breast milk is the optimal source of nutrition for all infants, especially those born preterm.19 Difficulties feeding and establishing breastfeeding are among the problems commonly found in MLPT newborns.
Some of the health problems that are more prevalent in MLPT newborns are associated with breastfeeding difficulties. A combination of factors predisposes these infants to inadequate intake during breastfeeding and their mothers to insufficient milk production.20
Breastfeeding in MLPT newborns requires increased supervision and support, as these infants have an immature sucking pattern, spend less time in the alert state, are less vigorous and have greater difficulty latching or sucking due to the size of their mouths, a lower oral motor tone and poor suck-swallow-breathe coordination, which results in decreased transfer of milk and hinders milk production due to insufficient stimulation. This contributes to difficulties in breastfeeding initiation and maintenance and to a higher proportion of early breastfeeding cessation, all of which is associated with increased maternal stress and anxiety.21
RecommendationsSee Table 3.
Management of breastfeeding and assessment of feeding difficulty in moderate to late preterm neonates.
| Breastfeeding and feeding difficulty |
|---|
| - Find out the infant feeding modality preferred by the mother after being appropriately informed. Assess her experience and knowledge (Grade C recommendation) |
| - Promote immediate skin-to-skin contact after birth and maintain it until at least the first feed in clinically stable MLPT neonates (Grade A recommendation. Level of evidence: Ia) |
| - Offer information and guidance regarding breastfeeding in preterm and/or low birth weight infants (e.g., sleep/alert states, hunger cues, feed duration). This is particularly important in primiparous mothers or those with past unfavorable experiences with breastfeeding or exposed to negative views about breastfeeding (Grade C recommendation) |
| - Initiate colostrum extraction early (6–12 h post birth), in every case, but particularly if separation of the mother and infant is necessary (Grade B recommendation. Level of evidence: III) |
| - Offer breastfeeding on demand, minimum of 10–12 feeds/day (Grade C recommendation) |
| - Maintain periodic expression of breast milk if mother and infant are separated. Until an adequate milk supply is established, recommend pumping after each feed until fully emptying the breasts (Grade C recommendation) |
| - Provide guidance on breast milk expression techniques (Grade C recommendation) |
| - Supplementary feeding may be necessary and should take place under medical prescription. Mother’s own milk is preferred for supplementation, and, if not available, donated human milk or formula can be used. Use a nursing supplementer or tube attached to the breast, the finger-syringe method or a bottle, depending on maternal skills and the anticipated infant feeding modality (Grade C recommendation) |
| - Establish a breastfeeding policy at the hospital known to the entire health care team. Ensure the staff knows and promotes the 10 steps to successful breastfeeding endorsed in the framework of the IHAN. (Grade A recommendation. Level of evidence: Ia) |
| - Breastfeeding assessment in at least one feed a day by a professional trained on breastfeeding (lactation consultant or IBCLC). Assess milk transfer and breast emptying. Assess the need for the temporary use of silicone nipple shields if sucking is inefficient or milk transfer is insufficient (Grade B recommendation. Level of evidence: IIb) |
| - Provide support and guidance for mothers who do not achieve adequate breastfeeding or choose not to breastfeed. Provide education on formula feeding including the correct preparation and administration of infant formula (Grade C recommendation) |
| - Daily monitoring of weight, transcutaneous bilirubin, urine output and bowel movements (Grade C recommendation) |
| - Close monitoring of infant feeding (frequency and duration of feeds, feeding modality, type and method of delivery of supplements if needed, weight, urine output, bowel movements and infant behavior) by qualified staff in the first 10–14 days post birth or until infant feeding has been adequately established (Grade C recommendation) |
| - Recommend continuation of kangaroo care at home after discharge. This is an effective and easy to implement method that promotes not only breastfeeding, but also the health and wellbeing of the infant and parents (Grade B recommendation. Level of evidence: III) |
Abbreviations: IBCLC, International Board Certified Lactation Consultant; IHAN, Iniciativa para la Humanización de la Asistencia al Nacimiento y la Lactancia (Spanish adaptation of baby-friendly hospital initiative [BFHI]); MLPT, moderate to late preterm.
The risk of hyperbilirubinemia is two to three times greater in MLPT compared to term newborns on account of immaturity and delayed development of hepatic bilirubin conjugation pathways. Feeding difficulties can delay the normalization of increased enterohepatic circulation of bilirubin.
RecommendationsSee Table 4.
Management of hyperbilirubinemia in moderate to late preterm neonates.
| Hyperbilirubinemia |
|---|
| - Establish maternal ABO and Rh blood type, Coombs test in mother and neonate (Grade C recommendation) |
| - Watch closely for the development of jaundice in the first 24 h post birth, and measure the total serum bilirubin if the infant is visibly jaundiced (Grade C recommendation) |
| - In infants born at or after 35 weeks’ gestation with birth weight of 2500 g or greater, apply the criteria established in the AAP guideline for initiation of phototherapy and exchange transfusion22 (Grade C recommendation) |
| - In infants born before 35 weeks’ gestation, follow the recommendations for initiation of phototherapy and exchange transfusion according to postmenstrual age published by Maisels et al.23 (Grade C recommendation) |
| - Serum or transcutaneous bilirubin measurement in all preterm infants within 72 h of birth (Grade C recommendation) |
| - Watch for the peak bilirubin level, which may occur later, between days 5 and 7 post birth. Late preterm infants discharged before day 5 post birth should be reevaluated between 24 and 72 h after discharge, including assessment of breastfeeding and weight gain and measurement of bilirubin (Grade C recommendation) |
| - Provide parents verbal and written information regarding neonatal jaundice, including: (Grade C recommendation) |
| • Signs and symptoms of jaundice |
| • Importance of visiting pediatrician if the baby appears to have jaundice |
| • Importance of maintaining adequate feeding/intake |
| • Importance of consulting with pediatrician if the baby is not feeding well |
Abbreviation: AAP, American Academy of Pediatrics.
Moderate to late preterm infants are at increased risk of neonatal infection, as maternal antibodies are not fully transferred before 37 weeks’ gestation. They may acquire early infection if preterm birth was triggered by an infection in the mother. They are also at increased risk of late healthcare-associated or community-acquired infection.
Recommendations- -
Intervene according to maternal carrier status for Streptococcus agalactiae24 (Grade B recommendation. Level of evidence: IIb)
- -
Identify perinatal risk factors. Establish detection strategies for patients at risk (blood screening, neonatal sepsis calculator or clinical observation) according to the protocols of the hospital25 (Grade C recommendation)
- -
Rigorous implementation of sterile and aseptic technique.
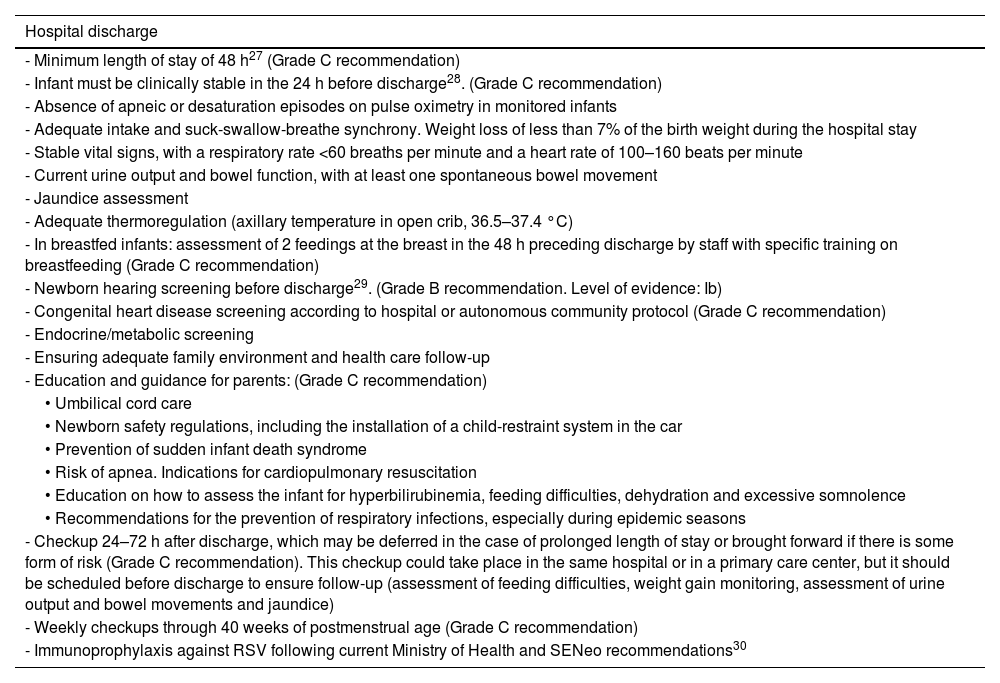
Most moderate preterm infants are admitted to the neonatal unit, so hospital discharge may be delayed a few weeks. Clinically stable late preterm infants are usually discharged a few days after birth, with a readmission rate 1.5–3 times greater compared to term infants and more frequent readmission within 15 days of discharge. The most frequent reasons for readmission in this subset of infants are jaundice, difficulty feeding, poor weight gain, dehydration, apnea and infectious diseases.26
In November 2022, the European Commission approved a new monoclonal antibody, nirsevimab, for the prevention of disease caused by RSV. In October 2023, Spain introduced the routine administration of nirsevimab to all infants aged less than 6 months (in the case of those born before 35 weeks’ gestation, 12 months) at the onset of the RSV season. Administration of nirsevimab is also indicated in children with a history of bronchopulmonary dysplasia, congenital heart defects or any other disease associated with increased risk in the case of RSV infection entering their second RSV season.
RecommendationsSee Table 5.
Criteria and recommendations for hospital discharge in moderate to late preterm neonates.
| Hospital discharge |
|---|
| - Minimum length of stay of 48 h27 (Grade C recommendation) |
| - Infant must be clinically stable in the 24 h before discharge28. (Grade C recommendation) |
| - Absence of apneic or desaturation episodes on pulse oximetry in monitored infants |
| - Adequate intake and suck-swallow-breathe synchrony. Weight loss of less than 7% of the birth weight during the hospital stay |
| - Stable vital signs, with a respiratory rate <60 breaths per minute and a heart rate of 100–160 beats per minute |
| - Current urine output and bowel function, with at least one spontaneous bowel movement |
| - Jaundice assessment |
| - Adequate thermoregulation (axillary temperature in open crib, 36.5–37.4 °C) |
| - In breastfed infants: assessment of 2 feedings at the breast in the 48 h preceding discharge by staff with specific training on breastfeeding (Grade C recommendation) |
| - Newborn hearing screening before discharge29. (Grade B recommendation. Level of evidence: Ib) |
| - Congenital heart disease screening according to hospital or autonomous community protocol (Grade C recommendation) |
| - Endocrine/metabolic screening |
| - Ensuring adequate family environment and health care follow-up |
| - Education and guidance for parents: (Grade C recommendation) |
| • Umbilical cord care |
| • Newborn safety regulations, including the installation of a child-restraint system in the car |
| • Prevention of sudden infant death syndrome |
| • Risk of apnea. Indications for cardiopulmonary resuscitation |
| • Education on how to assess the infant for hyperbilirubinemia, feeding difficulties, dehydration and excessive somnolence |
| • Recommendations for the prevention of respiratory infections, especially during epidemic seasons |
| - Checkup 24–72 h after discharge, which may be deferred in the case of prolonged length of stay or brought forward if there is some form of risk (Grade C recommendation). This checkup could take place in the same hospital or in a primary care center, but it should be scheduled before discharge to ensure follow-up (assessment of feeding difficulties, weight gain monitoring, assessment of urine output and bowel movements and jaundice) |
| - Weekly checkups through 40 weeks of postmenstrual age (Grade C recommendation) |
| - Immunoprophylaxis against RSV following current Ministry of Health and SENeo recommendations30 |
Abbreviations: RSV, respiratory syncytial virus; SENeo, Sociedad Española de Neonatología.
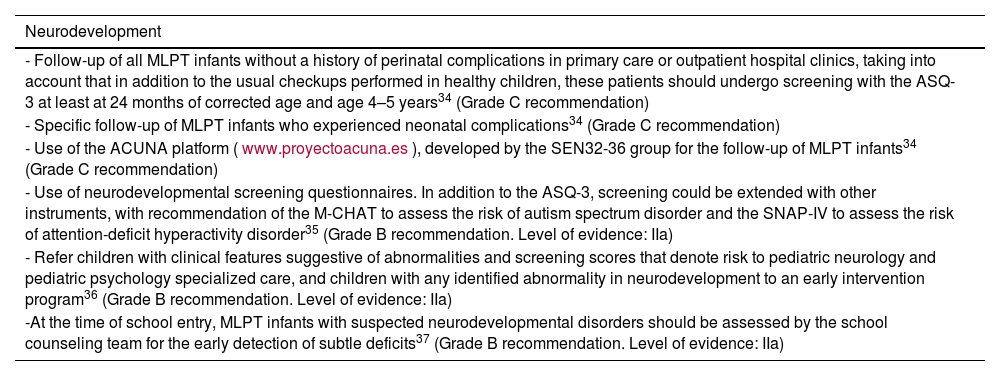
Moderate to late preterm infants exhibit a significant decrease in brain volume, less developed myelinization and gyral folding and alterations in brain white matter microstructure and connectivity.31 These factors probably contribute to the overall increased prevalence in these children of neurologic disease in the form of cerebral palsy, developmental delay or intellectual disability.32
Poorer executive function, visual-spatial perception and academic performance have also been reported in this population.33
RecommendationsSee Table 6.
Neurodevelopmental follow-up of moderate and late preterm infants.
| Neurodevelopment |
|---|
| - Follow-up of all MLPT infants without a history of perinatal complications in primary care or outpatient hospital clinics, taking into account that in addition to the usual checkups performed in healthy children, these patients should undergo screening with the ASQ-3 at least at 24 months of corrected age and age 4–5 years34 (Grade C recommendation) |
| - Specific follow-up of MLPT infants who experienced neonatal complications34 (Grade C recommendation) |
| - Use of the ACUNA platform (www.proyectoacuna.es), developed by the SEN32-36 group for the follow-up of MLPT infants34 (Grade C recommendation) |
| - Use of neurodevelopmental screening questionnaires. In addition to the ASQ-3, screening could be extended with other instruments, with recommendation of the M-CHAT to assess the risk of autism spectrum disorder and the SNAP-IV to assess the risk of attention-deficit hyperactivity disorder35 (Grade B recommendation. Level of evidence: IIa) |
| - Refer children with clinical features suggestive of abnormalities and screening scores that denote risk to pediatric neurology and pediatric psychology specialized care, and children with any identified abnormality in neurodevelopment to an early intervention program36 (Grade B recommendation. Level of evidence: IIa) |
| -At the time of school entry, MLPT infants with suspected neurodevelopmental disorders should be assessed by the school counseling team for the early detection of subtle deficits37 (Grade B recommendation. Level of evidence: IIa) |
Abbreviations: ASQ-3, Ages and Stages Questionnaires; M-CHAT, Modified Checklist for Autism in Toddlers; SNAP-IV, Swanson, Nolan, and Pelham Rating Scale-IV.
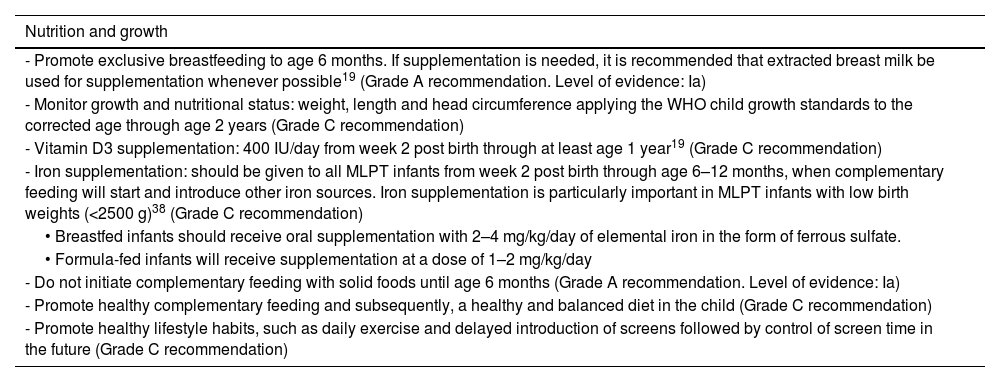
The nutritional status and growth of MLPT infants must be monitored19 and efforts made to prevent extrauterine growth restriction, given the impact it would have in their neurodevelopment, while also avoiding excessive weight gain that would increase the future risk of metabolic syndrome.
There are data suggesting that iron supplementation in these infants may have positive effects on growth and reduce anemia.38
Although osteopenia and vitamin D deficiency are less frequent compared to preterm infants born before 32 weeks’ gestation, vitamin D supplementation is recommended through at least age 1 year.19
RecommendationsSee Table 7.
Monitoring of nutrition and growth in moderate and late preterm infants.
| Nutrition and growth |
|---|
| - Promote exclusive breastfeeding to age 6 months. If supplementation is needed, it is recommended that extracted breast milk be used for supplementation whenever possible19 (Grade A recommendation. Level of evidence: Ia) |
| - Monitor growth and nutritional status: weight, length and head circumference applying the WHO child growth standards to the corrected age through age 2 years (Grade C recommendation) |
| - Vitamin D3 supplementation: 400 IU/day from week 2 post birth through at least age 1 year19 (Grade C recommendation) |
| - Iron supplementation: should be given to all MLPT infants from week 2 post birth through age 6–12 months, when complementary feeding will start and introduce other iron sources. Iron supplementation is particularly important in MLPT infants with low birth weights (<2500 g)38 (Grade C recommendation) |
| • Breastfed infants should receive oral supplementation with 2–4 mg/kg/day of elemental iron in the form of ferrous sulfate. |
| • Formula-fed infants will receive supplementation at a dose of 1–2 mg/kg/day |
| - Do not initiate complementary feeding with solid foods until age 6 months (Grade A recommendation. Level of evidence: Ia) |
| - Promote healthy complementary feeding and subsequently, a healthy and balanced diet in the child (Grade C recommendation) |
| - Promote healthy lifestyle habits, such as daily exercise and delayed introduction of screens followed by control of screen time in the future (Grade C recommendation) |
Abbreviation: WHO, World Health Organization.
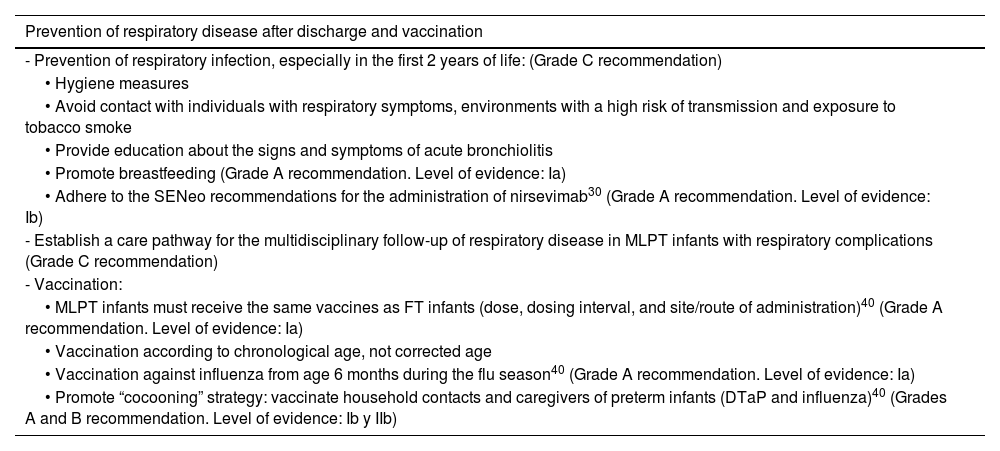
Respiratory problems are common in MLPT newborns in the short as well as the long term. There is evidence that lung function is reduced in MLPT infants with a history of moderate to severe respiratory disease in the immediate postnatal period.39
This negative respiratory effect in MLPT infants has a multifactorial etiology that also involves other factors, such as obstetric/fetal or neonatal disease and subsequent respiratory infections. Therefore, prevention is key.
In addition, preterm neonates are particularly vulnerable to infection due to the immaturity of the skin and mucosal barriers and the immune system, as well as the circumstances associated with their condition, which may also have an impact on the immune response. Infections, and respiratory infections in particular, are a significant cause of morbidity, mortality, medical visits and hospital readmission in this MLPT infants. This is why immunoprophylaxis is very important.40
RecommendationsSee Table 8.
Prevention of respiratory disease and vaccination.
| Prevention of respiratory disease after discharge and vaccination |
|---|
| - Prevention of respiratory infection, especially in the first 2 years of life: (Grade C recommendation) |
| • Hygiene measures |
| • Avoid contact with individuals with respiratory symptoms, environments with a high risk of transmission and exposure to tobacco smoke |
| • Provide education about the signs and symptoms of acute bronchiolitis |
| • Promote breastfeeding (Grade A recommendation. Level of evidence: Ia) |
| • Adhere to the SENeo recommendations for the administration of nirsevimab30 (Grade A recommendation. Level of evidence: Ib) |
| - Establish a care pathway for the multidisciplinary follow-up of respiratory disease in MLPT infants with respiratory complications (Grade C recommendation) |
| - Vaccination: |
| • MLPT infants must receive the same vaccines as FT infants (dose, dosing interval, and site/route of administration)40 (Grade A recommendation. Level of evidence: Ia) |
| • Vaccination according to chronological age, not corrected age |
| • Vaccination against influenza from age 6 months during the flu season40 (Grade A recommendation. Level of evidence: Ia) |
| • Promote “cocooning” strategy: vaccinate household contacts and caregivers of preterm infants (DTaP and influenza)40 (Grades A and B recommendation. Level of evidence: Ib y IIb) |
Abbreviations: DTaP, diphtheria, tetanus, and acellular pertussis vaccine; FT, full term; MLPT, moderate to late preterm; SENeo: Sociedad Española de Neonatología.
We thank Xavier Demestre, friend and teacher, first coordinator of the SEN34-36 group, now the SEN32-36 group, advocate and supporter of the assessment and follow-up of moderate and late preterm infants in Spain. We are grateful for his dedication and enthusiasm throughout these years.