Coeliac disease is a systemic immune-mediated disorder triggered by the ingestion of gluten, which is given in genetically predisposed subjects. It manifests with a wide variety of clinical symptoms, specific serological markers, HLA-DQ2/DQ8 haplotype, and enteropathy. The criteria followed for this have usually been those established by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) since 1969. These criteria have advanced from the need of several intestinal biopsies to, thanks to the development of serological tests of high sensitivity and specificity, considering the enteropathy as one more element in this diagnosis and makes it possible to perform a diagnosis without the need of an intestinal biopsy in certain circumstances. The updated review of the 2012 criteria in 2019 provides new evidence on some aspects, such as the role of HLA, the diagnosis of asymptomatic patients, and the effectiveness of the serological markers. These aspects are reviewed in detail, with the aim of facilitating the rational application of the new 2020 criteria at all care levels. In this sense, Paediatric Primary Care is fundamental in the search for active cases and to perform a first serological study, being recommended that the diagnosis is always established by a Paediatric Gastroenterologist.
La enfermedad celíaca es un proceso sistémico de carácter inmunológico, desencadenado por el consumo de gluten, que se da en sujetos genéticamente predispuestos. Se expresa con una gran variedad de síntomas clínicos, marcadores serológicos específicos, haplotipo HLA-DQ2/DQ8 y enteropatía. El tratamiento consiste en eliminar de por vida el gluten de la dieta, por lo que es fundamental un diagnóstico adecuado. Los criterios seguidos para ello han sido habitualmente los establecidos por la European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) desde 1969. Estos criterios han ido evolucionando desde la necesidad de varias biopsias intestinales para el diagnóstico a, gracias al desarrollo de pruebas serológicas de alta sensibilidad y especificidad, considerar la enteropatía como un elemento más en este diagnóstico y posibilitar en determinadas circunstancias realizarlo sin necesidad de biopsia intestinal. La revisión actualizada en 2019 de los criterios 2012 aporta nueva evidencia sobre algunos aspectos, como el papel del HLA, el diagnóstico de los pacientes asintomáticos y la eficacia de los marcadores serológicos. Estos aspectos se revisan en detalle, con el objetivo de facilitar la aplicación de los nuevos criterios 2020 de una forma racional en todos los niveles asistenciales. En este sentido el pediatra de Atención Primaria es fundamental para la búsqueda activa de casos y realizar un primer estudio serológico, recomendándose que el diagnóstico sea siempre establecido por un pediatra gastroenterólogo.
Based on the most recent definition of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN),1 coeliac disease (CD) is an immune-mediated systemic disorder elicited by the consumption of gluten and related prolamins (secalins, hordeins and possibly avenins) in genetically susceptible individuals (human leukocyte antigen [HLA] haplotype). It manifests with a variable combination of clinical symptoms, specific serological markers, a HLA-DQ2/DQ8 haplotype and enteropathy.
Treatment consists in the life-long strict elimination of gluten from the diet, which achieves resolution of symptoms, negative titres of autoantibodies and histological recovery of the intestinal mucosa.
Evolution of diagnostic criteriaIn 1969, the ESPGHAN established the first stringent criteria for diagnosis of CD in the paediatric population, the so-called Interlaken criteria2 (Table 1). They established as mandatory criteria evidence of villous atrophy in the initial intestinal biopsy (IB) while the patient consumes a gluten-containing diet, histological recovery after elimination of gluten and recurrence of the lesion after gluten reintroduction (challenge test) to differentiate CD from other causes of enteropathy.
Evolution of ESPGHAN criteria for diagnosis of CD.1,2,4,13
| 1969 criteria | 1990 criteria | 2012 criteria | 2020 criteria |
|---|---|---|---|
| • 3 IBs required for diagnosis. | • At least one initial IB is necessary for diagnosis. | • In symptomatic patients with an anti-tTG IgA titre >10 x ULN, positive EMA (in a second serum sample) and HLA DQ2/DQ8, CD can be diagnosed without the initial IB. | • In some asymptomatic patients meeting the established criteria, the diagnosis of CD can be made omitting the initial IB. |
| • Gluten challenge test required in almost every case. | • The gluten challenge test is required in all children aged less than 2 years or in case of uncertain diagnosis. | • The gluten challenge test is only required in case of uncertain diagnosis. | • HLA testing is not necessary for the no-biopsy diagnosis. |
| In patients with IgA deficiency or T1D, IB is required. | |||
| Time elapsed to confirmation of diagnosis based on the applied criteria: | |||
| • In all cases, diagnosis is confirmed after 5–6 years of followup. | • In most children, diagnosis is confirmed after 5–6 years of followup. | • In most cases, diagnosis is confirmed after 1–3 months of followup. | • In most cases, diagnosis is confirmed after 1–3 months of followup. |
CD, coeliac disease; EMA, endomysial antibodies; IB, intestinal biopsy; T1D, type 1 diabetes mellitus; tTG, tissue transglutaminase; ULN, upper limit of normal.
The application of these criteria along with the discovery in the early 1980s of a marker of active CD, antigliadin antibodies (AGAs),3 prompted their revision, leading to the establishment of the new 1990 ESPGHAN criteria for diagnosis of CD.4 These criteria allowed making the diagnosis in older children with symptoms suggestive of CD after a single IB with characteristic (albeit nonspecific) abnormal mucosal histology in the context of a gluten-containing diet and improvement of symptoms with exclusion of gluten from the diet. The gluten challenge was to be reserved for children aged less than 2 years at the time of the initial IB, cases diagnosed without an IB and cases of uncertain diagnosis.
Endomysial antibodies (EMAs)5 were discovered in the mid-1980s, and tissue transglutaminase (tTG) was identified in 1997 as the autoantigen of CD, followed by the discovery of the presence of circulating anti-tTG type 2 antibodies. Both types of antibodies had a similar sensitivity and specificity and performed better as markers of active CD than AGA.6
The 21st century: a new scenarioThe 1990 ESPGHAN criteria continued to be in use until numerous studies demonstrated the excellent correlation between anti-tTG antibody titres and the degree of villous6,7 and the strong performance of EMA titres for prediction of future lesions.8 This led to questioning the role of the traditional histological examination as the gold standard for diagnosis of CD8,9 and the need for a gluten challenge in children aged less than 2 years, as most of those with villous atrophy and positive EMA titres experienced a recurrence of symptoms with the challenge.9
As a result of the gathered evidence, the new ESPGHAN guidelines for the diagnosis for Coeliac Disease in children and adolescents. An evidence-based approach1 were published in 2012, with a novel definition of CD in which enteropathy was just one of the elements to consider in the diagnosis, and not a requisite criterion. The guidelines contemplated diagnosis without performance of an IB in certain cases (suggestive symptoms, high titres of anti-tTG antibodies, positive EMA antibodies in a second sample and compatible HLA haplotype). Evidence from the years that followed confirmed that diagnosis without a biopsy was safe in these cases,10–12 and the latest update of the 2012 guidelines, published in 2020, presents new evidence on certain aspects of CD, such as the role of HLA, the diagnosis of asymptomatic patients and the efficacy of serological markers.13 We will now review these aspects in detail with the aim of facilitating the rational application of the new 2020 criteria in the outpatient and inpatient settings.
Role of clinical manifestationsClinical signs and symptoms associated with CD (Table 2) are the features that, on appearing, lead to suspicion of CD and the need for differential diagnosis.
Situations in which CD should be investigated (adapted from the ESPGHAN 2020 guidelines).13
| In children and adolescents with any of the following symptoms if the aetiology is unknown: |
| • Gastrointestinal symptoms: |
| Chronic or intermittent diarrhoea |
| Abdominal distension |
| Recurrent nausea or vomiting |
| Chronic abdominal pain |
| Chronic constipation |
| • Extraintestinal symptoms |
| Failure to thrive, weight loss, stunted growth |
| Iron-deficiency anaemia |
| Abnormal results of liver function tests |
| Recurrent aphthous stomatitis |
| Short stature |
| Delayed puberty, amenorrhoea |
| Dermatitis herpetiformis |
| Chronic fatigue, irritability |
| Bone fracture from mild trauma/osteopenia/osteoporosis |
| Neuropathy |
| Arthritis, arthralgia |
| Dental enamel defects |
| In children and adolescents belonging to any of the following at-risk groups: |
| • First-degree relative with CD |
| • Diabetes mellitus type I |
| • Autoimmune thyroid disease |
| • Autoimmune liver disease |
| • Down syndrome |
| • Turner syndrome |
| • Williams syndrome |
The information available on the clinical presentation of CD has experienced drastic changes with the development of highly sensitive and specific serological tests. New categories of patients have been identified that have mild gastrointestinal symptoms, symptoms considered atypical (extraintestinal manifestations) or no symptoms, as well as factors that increase the risk of CD, which has led to changes in the clinical forms of disease currently diagnosed, with an increase in atypical and silent forms. Coeliac disease has gone from being considered a paediatric disease to being diagnosed throughout the lifespan.14
When it comes to the specificity of its features, a recent study in a cohort of patients with positive serological tests for CD found that the combination of a clinical picture of malabsorption (chronic diarrhoea, weight loss, failure to thrive and anaemia) and high titres of specific antibodies (anti-tTG antibody titre > 10 times the upper limit of normal [ULN] and a positive EMA result) had a 100 % positive predictive value for intestinal involvement compared to a slightly lower value if the antibody titres were combined with symptoms other than malabsorption.12
Screening for CD should also be performed in specific situations that carry an increased risk for it, such as autoimmune disease, certain chromosomal disorders, IgA deficiency and history of CD in a first-degree relative (Table 2).13
On the other hand, the notion of universal screening in the general population remains controversial, as CD does not fulfil the criteria established by the World Health Organization for screening tests. Different groups of experts support the recommendation of the ESPGHAN of active case finding, given the lack of evidence on the outcomes of asymptomatic patients that do not receive a diagnosis.15,16
Role of serologyGiven the high accuracy of the antibody tests used for diagnosis of CD, serological testing is recommended for initial screening of patients with suspected CD.
Antitransglutaminase antibodiesThe initial test in the evaluation of CD should be the anti-tTG antibody test on account of its high sensitivity and specificity as well as its wide availability and the use of an automated and objective method (enzyme immunoassay). A recent systematic review with meta-analysis16 found a sensitivity of 92.8 % (95 % confidence interval [CI], 90.3 %–94.8 %) and a specificity of 97.9 % (95 % CI, 96.4 %–98.8 %). Titres of anti-tTG IgA greater than 10 times the ULN predict the presence of villous lesions with a high specificity.17 There have been reports of occasional false positive results, usually at low titres, due to cross-reactivity with antibodies present in other clinical conditions, such as autoimmune diseases, liver diseases or infections.18
Endomysial antibodiesEndomysial antibodies react with the endomysium, the perivascular connective tissue that lines smooth muscle bundles. They target transglutaminase in tissues such as monkey oesophagus or umbilical cord tissue, which are used as substrates in the tests developed for their measurement. The diagnostic accuracy of EMA IgA is very high,19 with a high sensitivity and specificity.13 The main drawback of this marker is that it is measured by means of indirect immunofluorescence, a semiquantitative and subjective method that is more expensive, requires experienced staff and takes longer than the measurement of anti-tTG antibodies.
Antibodies against deamidated forms of gliadin peptidesGliadin peptides are deamidated by tissue transglutaminase, which results in a significant increase in their immunogenicity. This inspired the development of methods for detection of deamidated gliadin peptide (DGP) antibodies, which have since replaced previously used tests for detection of antigliadin antibodies. One advantage of DGP antibodies is that they are detected by an objective method, enzyme immunoassay. The sensitivity of DGP IgA and IgG antibodies is 87.8 % (95 % CI, 85.6 %–89.9 %) and 94.1 % (95 % CI, 92.5 %–95.5 %), respectively.16 Testing for DGP IgG antibodies is useful for evaluation of CD in patients with selective IgA deficiency.20 However, the yield is lower compared to positive anti-tTG IgA titres and isolated positive DGP results may give rise to false positives that increase the frequency of unnecessary endoscopies.21–23
Rapid testsThey are performed by means of immunochromatography, and therefore are not quantitative. They involve application of few drops of capillary blood on a solid support, and as the blood diffuses through the matrix a line becomes visible in the test window if the result is positive. There are rapid tests for detection of anti-tTG type 2, AGA or a combination of several antibodies. These tests have been used for non-invasive screening of CD and exhibited a good correlation with standard serological tests as long as the tests are interpreted by adequately trained staff. The sensitivity and specificity of these tests are 94.0 % (95 % CI, 89.9 %–96.5 %) and 94.4 % (95 % CI, 90.9 %–96.5 %) respectively, although currently there are not enough data to support their use in everyday clinical practice, so their results must always be confirmed by standard serological testing.24
Limitations of serological testsAlthough the measurement of anti-tTG IgA antibodies is not standardised, most commercially available tests are highly accurate, especially at high titres.25 However, there is evidence of variability between different tests or different laboratories using the same test when it comes to moderate anti-tTG titres.12 Laboratories must be extremely rigorous in their internal quality control measures, accurately calculating the calibration curve, which should include the value of 10 times the ULN.13
Serological testing must be performed while the patient continues to consume gluten, as antibody titres decrease after initiation of a low-gluten or gluten-free diet. If a patient was on a gluten-restricted diet during the diagnostic evaluation, experts recommend consumption of a normal diet including at least 3 slices of bread a day for 1–3 months to then repeat the measurement of anti-tTG antibodies.26
The initial anti-tTG IgA measurement should be accompanied by measurement of total serum IgA and, in case of detection of selective IgA deficiency, the patient should be further evaluated with performance of anti-tTG, EMA or PDG IgG tests.1,13 In contrast to anti-tTG IgA titres, no studies have been conducted to establish the levels of anti-tTG IgG that can reliably predict the presence of enteropathy. Thus, performance of an IB is necessary in these children to confirm the diagnosis.13 Other possible causes of false negatives are immunosuppressive therapy and dermatitis herpetiformis, usually associated with negative serology. Measurement in haemolysed specimens may also reflect a false decrease in antibody titres.27
The evidence is also insufficient as regards the correlation of high anti-tTG titres with the presence of enteropathy in children with type 1 diabetes (T1D). In addition, high titres of anti-tTG antibodies have been described in stage 1 of T1D with spontaneous normalization of CD serology at moderate titres,28,29 so performance of an IB is recommended for diagnosis of CD in these patients.
Role of genetic testingCoeliac disease is associated with a specific high-risk variants of the HLA class II region, whose alleles encode molecules involved in the presentation of gluten peptide antigens to CD4+ T cells, thus playing a role in the immune cascade that results in gluten intolerance. More than 90 % of patients with CD are found to be homozygous or heterozygous for the HLA DQ2.5 variant encoded by the DQA1*05 (α-chain) and DQB1*02 (β-chain) genes. The isolated HLA-DQ2.2 haplotype (DQA1*02-DQB1*02) carries a much lower risk, for while the resulting heterodimer is homologous to the DQ2.5 heterodimer it forms less stable complexes with the peptides. Patients that do not have the DQ2 type have the DQ8 type, encoded by the DQA1*0301-DQB1*0302 alleles.1 There are several combinations of the 2 alleles that carry a specific risk of developing CD.30 Class II HLA genes account for 40 % of the genetic risk of CD, while other parts of the genome have been shown to have an influence of less than 10 %.31 In recent years, the complexity of the interpretation of HLA typing has led to efforts to standardise the nomenclature.32
High-risk HLA haplotypes are relatively frequent in some populations, which is associated with a higher incidence of CD if consumption of wheat and other grasses is customary in the population, as is the case in Spain.30 In this sense, HLA genotyping is of little use for diagnosis, as it has a very low positive predictive value. However, its high negative predictive value makes it very useful in ruling out CD in case of uncertain diagnosis and to establish the risk of developing CD in population groups with a high prevalence of the disease.12,19,33,34
Recent studies have reported that nearly 100 % of individuals with anti-tTG antibody titres above 10 times the ULN, a positive EMA result and an abnormal mucosa have a DQ2/DQ8 haplotype, which means that HLA typing would not be necessary to confirm the diagnosis without an IB,12,19 as only individuals with DQ2/DQ8 haplotypes can produce these antibodies.35
Due to all of the above, it seems reasonable to consider that the interpretation of the HLA haplotype and the associated risk is just one among many tools in the management of CD and needs to be performed by paediatricians specialised in paediatric gastroenterology and with experience in the management of CD,1,13 and therefore we do not recommend HLA genotyping for initial screening of CD at the primary care level.
Role of intestinal biopsyAn intestinal biopsy is no longer required for diagnosis of CD in every patient,1 but it unarguably continues to be an essential tool.
The interpretation of histological findings poses some challenges and therefore may vary between pathologists. To optimise the analysis of the intestinal mucosa, we recommend obtaining several biopsy samples through endoscopy, with at least 4 specimens of duodenal mucosa and 1 of the duodenal bulb, as in some cases this is the only affected area.1,13
A standardised protocol must be used to obtain correctly oriented cuttings of duodenal tissue. A villus height and crypt depth ratio of less than 2 in at least 1 of the biopsy samples is considered diagnostic of CD.36 The scheme used most widely to classify the degree of mucosal injury is the Marsh classification modified by Oberhüber37 (Table 3). The diagnosis of CD requires the presence of Marsh 2 or 3 histological changes, although no degree of enteropathy is pathognomonic.
Marsh–Oberhüber classification.37
| Stage 0 Preinfiltrative | Histologically normal mucosa | ||
| Stage 1 Infiltrative | Increased IELs with preserved villous architecture | ||
| Stage 2 Hyperplastic | Hyperplastic crypts and increased IELs | ||
| Stage 3 Destructive | Villous atrophy with hyperplastic crypts and increased IELs | Subdivision stablished by Oberhüber based on the degree of VA: | |
| 3a partial VA | |||
| 3b subtotal VA | |||
| 3c total VA | |||
| Stage 4 Hypoplastic | Irreversible villous atrophy | Does not respond to gluten-free diet, screen for establishment of malignant T-cell clone in the intestinal tract. |
IEL, intraepithelial lymphocyte; VA, villous atrophy.
It is important to include the following information in the pathology report:
- •
Whether the amount of tissue in the sample was sufficient
- •
Whether the specimens were well oriented and allowed assessment of villous height
- •
Percentage of intraepithelial lymphocytes (IELs) in relation to the total epithelial cell count
- •
Presence of absence of crypt hyperplasia (mitosis)
- •
Degree of villous atrophy.
The presence of Marsh 1 changes (a density of more than 25 IELs per 100 epithelial cells) is not sufficient to diagnose CD, as this amount of injury can be attributed to other causes, such as food allergy, infection (viral aetiology, Giardia, Cryptosporidium, Helicobacter pylori), drugs, autoimmune disease, inflammatory bowel disease, etc.38 Mild changes can also be found in patients with possible CD (patients with mild Marsh o or 1 changes and positive anti-tTG and EMA results combined with a high-risk HLA haplotype), in whom prescription of a gluten-free diet is controversial.39 In these cases, it may be useful to analyse the cytometric pattern of IEL subpopulations (elevation of CD3+TCRαβ T cells associated with a decrease in NK-like cells) or the presence of anti-tTG antibody deposits in the mucosa.13,39
The above notwithstanding, in some cases there is still disagreement between the results of anti-tTG antibody testing and histological findings. In such cases, we recommend a histological re-evaluation by examination of additional biopsy specimens or getting a second opinion from an expert pathologist.13
European Society for Paediatric Gastroenterology, Hepatology and Nutrition 2012 criteriaIn light of the excellent correlation found between anti-tTG and EMA IgA antibody test results and the presence of mucosal changes, the 2012 ESPGHAN guidelines1 allowed the possibility of diagnosing CD without an IB exclusively in patients meeting the following criteria:
- •
Suggestive symptoms
- •
Anti-tTG IgA titres greater than 10 times the ULN
- •
Positive EMA IgA test, which has the highest specificity, in a second serum sample to confirm the diagnosis and minimise the impact of potential methodological or labelling errors in the initial measurement of anti-tTG IgA titres.
- •
Compatible HLA-DQ haplotype (DQ2 and/or DQ8).
The accuracy of this approach was confirmed in 2 large-scale studies published in 2017.12,19 In everyday clinical practice, this approach prevents at least 35 % of IBs, mainly in children aged less than 5 years and in patients with significant symptoms.40
In recent years, the role of serological testing and the 2012 ESPGHAN criteria for diagnosis of CD has been analysed in scenarios other than the symptomatic patient. Evidence has emerged that anti-tTG IgA titres greater than 10 times the ULN predict the presence of mucosal injury in asymptomatic patients, although the positive predictive value of this finding may be lower compared to symptomatic patients.12,13
Updated 2020 criteriaDue to all of the above, the 2020 guidelines13:
- •
Confirm that the initial evaluation of CD should involve measurement of anti-tTG IgA and total serum IgA levels. In case of IgA deficiency, the second step should be testing for IgG antibodies.
- •
Allow the option of diagnosing CD in asymptomatic patients without an IB following the same steps used in symptomatic patients. However, since the positive predictive value of high anti-tTG titres is lower in asymptomatic patients, the decision to use the no-biopsy approach should be made on a case-by-case basis and with the agreement of the parents and also the patient if age allows.
- •
Do not allow the no-biopsy approach for diagnosis of CD in asymptomatic patients with T1D, as the scientific evidence in this patient subset is currently insufficient.
- •
Maintain the need for an IB in patients with IgA deficiency due to the lack of data on the predictive value of IgG antibodies for the presence of mucosal injury.
- •
Establish that HLA genetic testing is unnecessary in patients that require an IB or with anti-tTG IgA titres greater than 10 times the ULN. This test would only be indicated for screening of at-risk individuals and in case of uncertain diagnosis.
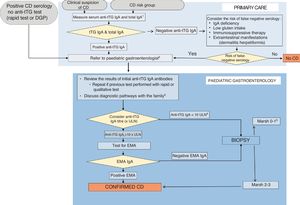
The evolution of the ESPGHAN criteria for diagnosis of CD a in the past 50 years has led to the current recommendations, with 7 core recommendations (Table 4). The process to follow in case of suspected CD or presence of risk factors is summarised in a diagnostic algorithm (Fig. 1). Primary care paediatricians play an essential role in suspecting the diagnosis and ordering the initial serological tests. The ESPGHAN recommends that the diagnosis always be made by a paediatric1,13 given that a diagnosis of CD involves the prescription of a gluten-free diet for life, and, in most cases, the diagnosis is confirmed within a short time frame, all with the ultimate purpose of avoiding both overdiagnosis and underdiagnosis and their consequences (Table 5).41 At the primary care level, this calls for maintenance of a gluten-containing diet until the diagnosis is confirmed.
Core recommendations of the ESPGHAN for diagnosis of CD in children and adolescents; 2012 and 2020 guidelines.1,13
| • CD should be suspected in children presenting any of the signs, symptoms of risk factors listed in Table 2. |
| • The first step in diagnosis is measurement of serum levels of total IgA and anti-tTG IgA antibodies. |
| • In the absence of CD antibodies (anti-tTG or EMA), the probability of CD in children is extremely low |
| • The diagnosis of CD can be established without an IB in children and adolescents with symptoms suggestive of CE and an anti-tTG IgA titre > 10×ULN confirmed by a positive EMA test (in a second sample) in a paediatric gastroenterology unit. |
| • The same no-biopsy diagnostic protocol may be applied to asymptomatic children, but this must be considered individually in each case must in a paediatric gastroenterology unit. |
| • In asymptomatic children with IgA deficiency or T1D, an IB is required to confirm the diagnosis. |
| • Individuals with HLA haplotypes other than DQ2 /DQ8 have a very low risk of developing CD. HLA testing is not required to make the diagnosis without an IB in patients with an anti-tTG IgA titre >10×ULN confirmed by a positive EMA test. HLA typing is indicated for screening in individuals with risk factors or an uncertain diagnosis. |
CD, coeliac disease; EMA, endomysial antibodies; IB, intestinal biopsy; T1D, type 1 diabetes mellitus; tTG, tissue transglutaminase; ULN, upper limit of normal.
Diagnostic algorithm (adapted from ESPGHAN 202013).
1. IgA deficiency: values below the thresholds established by the laboratory for age or < 0.2g/L in children aged more than 3 years.
2. Ask the family not to initiate a low-gluten or gluten-free diet before the patient is evaluated in the Paediatric Gastroenterology department.
3. Convey the message that regardless of how the diagnosis is reached, treatment with a gluten-free diet is life-long.
4. In case of a positive anti-tTG IgA test with a low titre, confirm sufficient dietary gluten intake. Consider repeating serological tests adding the EMA test.
5. Consider: a. revision of biopsy results; b. false positive anti-tTG result and testing for EMA (if positive EMA=potential CD); c. additional tests (HLA, anti-tTG deposits, cytometry, etc); d. consider followup and retesting ensuring normal gluten intake; e. assess the relevance of symptoms.
tTG=tissue transglutaminase antibodies, EMA=endomysial antibodies; DGP=deamidated gliadin peptide antibodies, ULN=upper limit of normal.
Consequences of errors in the diagnosis of CD.41
| Underdiagnosis (maintenance of gluten in the diet) | Overdiagnosis (unnecessary elimination of gluten) |
|---|---|
| Impact on quality of life due to persistent symptoms | Impact on quality of life of patient and family |
| Risk of impaired growth and development | Economic impact |
| Long-term complications: | Risk of imbalanced diet: potential deficiencies in vitamins and trace minerals |
| Reproductive problems/osteopenia | Risk of constipation and overweight |
| Gastrointestinal malignancy | |
| Autoimmune disease |
The authors have declared no conflicts of interest.
Please cite this article as: Riechmann ER, Villasante GCd, Pascual MLC, Aliaga ED, Allué IP, Sánchez-Valverde F, et al. Aplicación racional de los nuevos criterios de la European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) 2020 para el diagnóstico de la enfermedad celíaca. An Pediatr (Barc). 2020;92:110.