Due to its severity, as well as the consequences of a late diagnosis, critical congenital heart defects (CCHD) represent a challenging situation, making an early diagnosis necessary and ideally before symptoms appear when circulatory collapse or death of the newborn can occur.
Due to this, a prenatal and very early postnatal diagnosis is very important. Prenatal ultrasound screening and physical examination of the newborn can miss a considerable number of CCHD cases. Pulse oximetry screening has been demonstrated to be an effective, non-invasive, inexpensive, and well accepted tool in the early diagnosis of CCHD.
The Spanish National Society of Neonatology, through its Standards Committee, and based on the current evidence, recommend the implementation of pulse oximetry screening of CCHD in Spain, and then to offer the best therapy possible to these newborn infants.
Debido a su gravedad y a las consecuencias de un diagnóstico tardío, los defectos cardíacos congénitos críticos (DCCC) representan un reto, por lo que es necesario su diagnóstico muy precoz, idealmente antes del comienzo de los síntomas clínicos, que normalmente preceden al colapso circulatorio o muerte del recién nacido.
Por ello es importante su diagnóstico prenatal y posnatal muy precoz; sin embargo, tanto el diagnóstico por ecocardiografía foetal como la exploración física del recién nacido pueden ser insuficientes para diagnosticar un número importante de estos DCCC. El cribado de DCCC mediante el uso de pulsioximetría ha demostrado ser un método eficaz, no invasivo y de bajo coste, además de bien tolerado, para detectar a recién nacidos asintomáticos y afectos de DCCC en las primeras horas después del nacimiento.
La Sociedad Española de Neonatología, a través de su Comisión de Estándares, hace una recomendación, basada en la evidencia actual, para la implementación en nuestro medio de la pulsioximetría como cribado neonatal de DCCC, y poder ofrecer a estos recién nacidos el mejor tratamiento posible en cada caso.
In 2011, the United States Department of Health recommended the use of pulse oximetry (PO) for screening of critical congenital heart defects (CCHDs),1 followed by the development of consensus guidelines by a work group.2,3 Providers from different countries are advocating for recommending such screening in Europe.4
This document of the Standards Committee of the Sociedad Española de Neonatología (Spanish Society of Neonatology) endorses the recommendation of screening full term or late preterm newborns who are asymptomatic and do not require admission to a neonatal unit. The purpose of this measure is to reduce the risk of delays in the diagnosis of CCHDs, defined as congenital heart defects requiring invasive intervention or resulting in death in the first 30 days of life.5
MethodsWe conducted a systematic review, searching for sources by means of MeSH and free text terms in the Medline and ISI Web of Knowledge databases.
We determined the quality of the evidence and the strength of recommendation according to the levels of evidence established by the Oxford Centre for Evidence-Based Medicine (http://www.cebm.net) and the grades of recommendations established by the Canadian Task Force on Preventive Health Care.6
Rationale for screeningThe incidence of moderate and severe congenital heart defects is of 6 in 1000 live births (19/1000 with the inclusion of bicuspid aortic valve), while the overall incidence of congenital heart disease is of 75 in 1000 live births.7
The reported incidence of CCHDs ranges between 2.3 cases in 1000 live births and 1 case in 26,000 live births (25% of the total).5,7 The diagnosis of CCHDs is delayed in 30% of cases.8
There is evidence that the sensitivity of foetal echocardiography is low (68.1%; 95% CI, 59.6–75.5).9 While the diagnostic yield of prenatal echocardiography may be higher in some facilities, their experience cannot be generalised,10,11 and a study conducted in a population similar to that of Spain found that only 60% of cases of CCHDs were detected prenatally by this method.12
The physical examination fails to detect up to 20–30% of CCHDs.13,14 Heart murmurs are not always present in CCHDs, and may occur in up to 60% of healthy newborns.15,16 Visual assessment of newborn colour is not effective for the detection of hypoxaemia.17,18
The combination of prenatal ultrasound and physical examination may fail to detect between 29.5%8 and 20%12 of CCHDs.
Pulse oximetry for screening of critical congenital heart defectsA 2012 systematic review on this subject included 13 studies with data for 229,421 newborns. It found a sensitivity of PO screening of 76.5%, a specificity of 99.9%, and a false positive rate of 0.14%.19 The reviewers concluded that PO met the criteria to for universal screening of CCHDs.
Furthermore, PO screening has been well accepted by both health care professionals and families.20
There is sufficient evidence to recommend neonatal screening by PO in the first hours post birth, in addition to prenatal ultrasound and the physical examination (level of evidence A).
Timing of screeningWhen screening is performed 24h post birth, the false positive rate can drop to as low as 0.05%, while if performed in the first 24h after birth, the rate is as high as 0.50%.19
False positives may be indicative of other diseases, making these false positives “diagnostic.”
An analysis of late screens (>24h) demonstrated that half of CCHDs manifest in the first 24h and 20% do so with cardiovascular compromise.21
A retrospective review of screens performed before 12h post birth (applying a UK protocol) detected CCHDs in 9 out of 26,000 live births, with a false positive rate of 0.8%. However, 79% of false positives corresponded to patients with clinically significant conditions that required urgent intervention. The remaining 21% had transitional circulation (real false positives).22
The Nordic guidelines for pulse oximetry screening23 recommend its performance before 24h. Half of the patients in who screen results may have been positive develop symptoms before the screen is performed.12,24
When it comes to home birth, there is evidence of the feasibility of PO screening in the early hours post birth, with a median time point of 1.8h post birth for the first measurement (interquartile range, 1.3–2.8h) and 37h for the second measurement (interquartile range, 27–47h), and a 1% false positive rate. A high proportion of families find home screening acceptable.25,26
Based on the available evidence, early screening (before 24h post birth) is recommended over late screening (after 24h), with screening being most effective when performed in the first 12h post birth, despite the increase in the number of false positives (level of evidence B).
Sites of testingAlthough the aforementioned meta-analysis did not find significant differences in sensitivity between studies that used postductal saturations alone and those that used pre- and postductal saturations,19 the combination of pre- and postductal saturations increases the detection of left ventricular outflow tract abnormalities.27
Measurement of pre- and postductal saturations achieves detection of a greater number of CCHDs, equivalent to 7 in 100,000 births, although it can also increase the number of false positives compared to single postductal measurements.24,27
Therefore, the combination of pre- and postductal measurements increases the number of CCHDs detected through screening, with the disadvantage of being more time-consuming (level of evidence B).
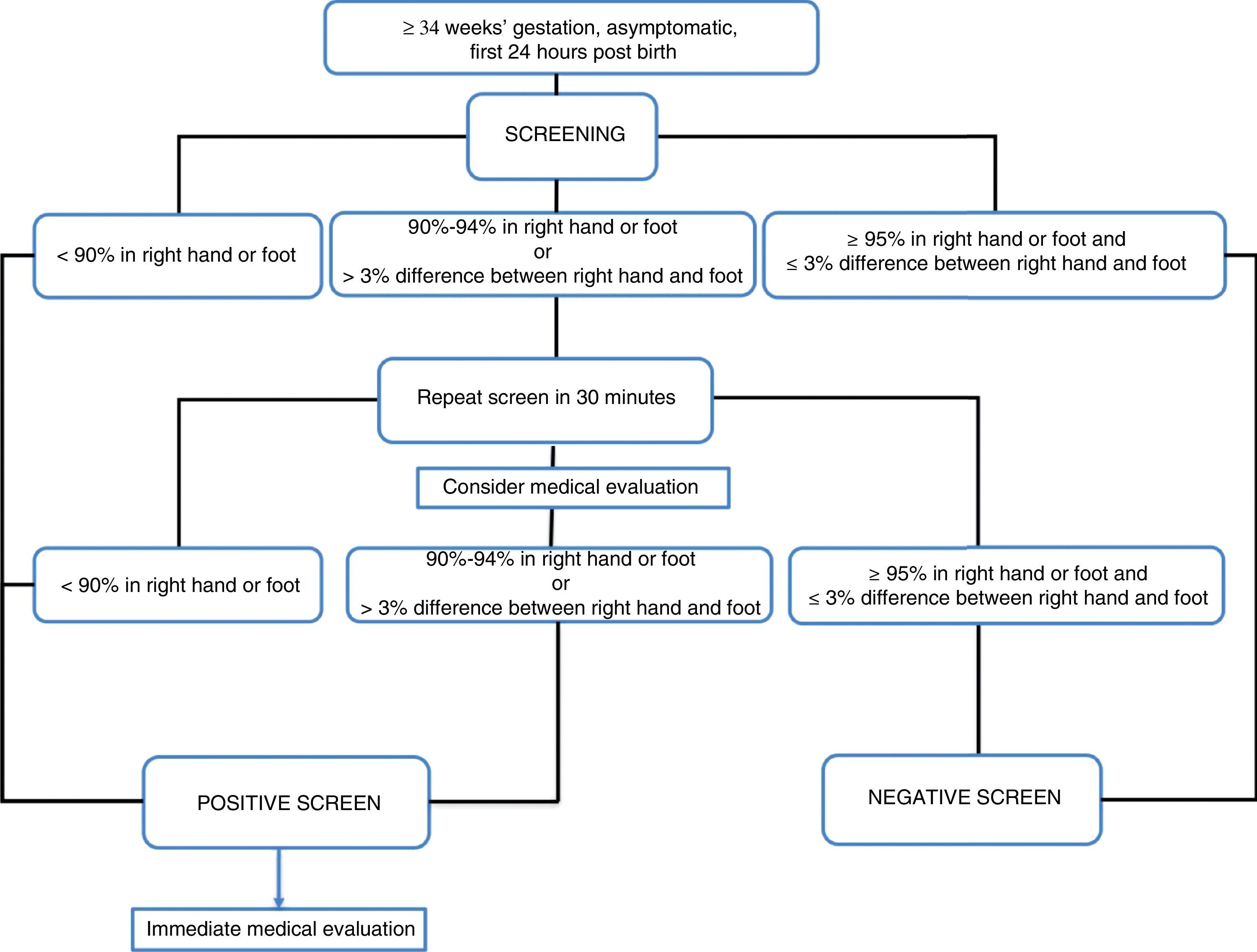
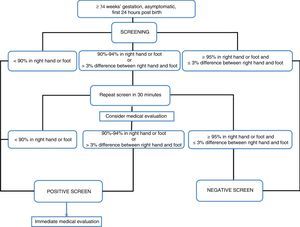
Definition of a positive resultPulse oximetry algorithms establish a saturation threshold of 95%, with saturations of less than 90% considered a strong positive and saturations between 90% and 94% considered a weak positive.
The American Academy of Pediatrics defines a positive screen as a saturation of less than 90% in any extremity, or between 90% and 94% in both the upper and lower extremities, or a difference between hand and foot saturations greater than 3%, on three different measurements, each separated by 1h, before performing a medical evaluation.3
The algorithm used in the United Kingdom defines a positive screen as a saturation of less than 90% at any time, or ranging between 90% and 94% in the right hand or either foot, or a difference in saturation of more than 2% between the hand and foot, in 2 different measurements 2h apart, as long as the patient is asymptomatic. In the presence of symptoms, the screen is considered positive.27
In the Nordic algorithm,23 the screen is positive when saturation in either extremity is less than 90%, or the saturation is between 90% and 94% in both extremities or the difference between the two is greater than 3% in three separate occasions at 30min intervals.
Thus, the optimal value to define test negativity is 95% saturation or higher, with a strong positive defined as a saturation of less than 90% or the presence of symptoms irrespective of oxygen saturation (level of evidence B),19 and it is reasonable to apply the criteria for positivity of saturation less than 95% in either extremity or a difference between hand and foot of more than 3% (level of evidence B).
There is disagreement in the interpretation of saturations between 90% and 94%. There are two sets of criteria for considering them positive: when the saturation is less than 95% in both extremities or the difference between the two extremities is greater than 3%,24 and when the saturation is less than 95% in either extremity or the difference between the two extremities is greater than 2%.28 The false positive rate is higher using the first approach as opposed to the second (0.15% vs 0.8%).
There is no consensus as to the clinical approach to take and the need for retesting when the measured saturation is between 90% and 94%. The potential advantage of waiting and repeating the test is the reduction in the number of false positives, and the disadvantage is the delay in diagnosis.
We think that a reasonable approach to a saturation of 90–94% is to perform a medical assessment and repeat the test only once (level of evidence B).
Devices to be usedScreening should be performed with oximeters appropriate for use in newborns that are motion-tolerant and with a high sensitivity under low saturation conditions (level of evidence B). We also recommend the use of next-generation equipment.1,3,29 This is important in the measurement of saturations of more than 70%.30
Expected impact of screeningFor screening performed within 24h, we estimate a hospital admission rate of 6.25 admissions per 1000 births22 and performance of 9 additional echocardiograms per 18,801 screens (42 months of screening).31
Cost-effectiveness of screeningMost studies conclude that PO screening of CCHDs is cost-effective. Overall, the estimated cost of each test is of 3.88 to 14 US dollars, with a total cost of screening per patient diagnosed with CCHD of 46,300 US dollars.32–34
Estimated duration and cost of screeningThe screening procedure generally takes 5.5–9min to complete, and can be performed by the staff scheduled on the floor without need for further personnel.32,34
Recommendations- 1.
A protocol for the postnatal screening of CCHDs in healthy, asymptomatic, non-hospitalised newborns needs to be implemented (A).
- 2.
Pulse oximetry is useful and safe for CCHD screening in newborns (A).
- 3.
The timing of screening affects its sensitivity, with a higher sensitivity the earlier it is performed (A).
- a.
Early screening, within 24h of birth, reduces the risk of onset with severe or very severe symptoms in CCHD at the expense of a greater number of false positives, although most of the latter are indicative of other disorders that may also require observation, diagnosis and treatment, so early screening is preferable to late screening (>24h).
- b.
Very early screening (<12h) may result in an excessive number of false positives, an issue that needs to be weighed at the local level.
- c.
In case of very early discharge, screening should be performed before discharge, regardless of timing.
- d.
It is recommended that the screen be performed between 6 and 24h post birth (B).
- a.
- 4.
Home birth does preclude performance of CCHD screening (B).
- 5.
Measurement of pre- and postductal saturations by PO is recommended to improve the diagnostic yield of screening (B).
- 6.
Screening should be performed with motion-tolerant oximeters intended for use in newborns and validated in low-perfusion conditions (B).
- 7.
A strong positive would correspond to a saturation <90% in the right hand or the foot or the presence of symptoms. In either situation, the newborn should undergo an appropriate medical evaluation (B).
- 8.
A strong negative would correspond to a saturation of ≥95% in the right hand and the foot, or a saturation ≥95% in any extremity with a difference between the two sites ≤3% (B).
- 9.
If the saturation is of 90–94% in the right hand or the foot, or the difference between them is greater than 3%, the measurement should be repeated in 30min (B). A medical evaluation is recommended at this point (B).
- 10.
If after the second measurement the newborn continues to have a saturation of 90–94% in the right hand or the foot, or the difference between the two is greater than 3%, the screen should be considered positive (B).
- 11.
Basic training should be given to the staff in charge of performing the screens (B).
Fig. 1 presents the algorithm for critical congenital heart defect screening recommended by the Standards Committee of the Sociedad Española de Neonatología.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sánchez Luna M, Pérez Muñuzuri A, Sanz López E, Leante Castellanos JL, Benavente Fernández I, Ruiz Campillo CW, et al. Cribado de cardiopatías congénitas críticas en el periodo neonatal. Recomendación de la Sociedad Española de Neonatología. An Pediatr (Barc). 2018;88:112.e1–112.e6.