Late prematures (LP) belong to a subgroup of many premature babies with a risk of delayed psychomotor development (PMD). Many subtle changes pass unnoticed if adequate assessment tools are not used. The Ages & Stages Questionnaires 3® (ASQ3®) for parents appears simple and useful for the detection of risk of impairment of PMD, and is recommended by scientific societies that study LP.
ObjectivesTo evaluate the risk of impaired PMD in LP at 5 years-old, and compare them with term newborns (TNB) using the ASQ3.
Patients and methodsData were collected on the LP born in a third level hospital in 2010, as well as 2 TNB of the same gender for each LP. The prenatal and postnatal morbidity variables were compared. At 5 years, their families (excluding those with other neurological risks) were asked to complete the ASQ3. The cut-off point was determined for the total score of the ASQ3 that would discriminate the risk of PMD impairment using ROC analysis. The cut-off point to determine a change in each domain was obtained according to the ASQ3 manual.
ResultsThe ASQ3 was completed for 88 (47%) and 131 (35%) TNB. All the overall mean scores and those for domains were lower in LP, with no significant differences found between the two groups. A risk of PMD impairment (≤253 points) was observed in 7 LP compared to 4 TNB, with no significant difference. More maternal, foetal, and neonatal illnesses were observed in 195 LP than in the 390 TNB. In the univariate analysis, male gender and restricted uterine growth (RUG) were factors associated with a risk of PMD impairment and only RUG in the multivariate analysis.
ConclusionThe risk of PMD impairment between LP and TNB at 5 years appears not to be shown, with no significant differences between both, and with the values obtained in the ASQ3 being slightly lower in the LP. Male gender and RUG negatively influence this risk.
Los prematuros tardíos (PT) son un subgrupo de prematuros numeroso con riesgo de déficit en el desarrollo psicomotor (DPM). Muchas alteraciones sutiles pasan desapercibidas si no se emplean herramientas adecuadas de evaluación. El cuestionario para padres Ages & Stages Questionnaires3® (ASQ3®) parece sencillo y útil para la detección de riesgo en el DPM y está recomendado por sociedades científicas que estudian a los PT.
ObjetivosEvaluar el riesgo de déficit en el DPM a los 5años de edad en PT y compararlos con recién nacidos a término (RNAT) con ASQ3.
Pacientes y métodosSe recogieron los PT nacidos en un hospital de tercer nivel en el año 2010 y 2RNAT del mismo sexo por cada PT. Se compararon variables de morbilidad prenatal y neonatal. A los 5años se solicitó a sus familias (excluyendo aquellos con otros riesgos neurológicos) completar ASQ3. Se determinó el punto de corte para la puntuación total de ASQ3 que discriminará el riesgo de déficit del DPM mediante un análisis ROC. El punto de corte para determinar alteración en cada dominio se obtuvo según el manual ASQ3.
ResultadosASQ3 fue completado por 88PT (47%) y 131RNAT (35%). Todas las puntuaciones medias globales y por dominios fueron menores en PT, sin encontrar diferencias significativas entre ambos grupos. Siete PT tuvieron riesgo de déficit del DPM (≤253 puntos) frente a 4RNAT, sin diferencia significativa. Un total de 195PT tuvieron mayor patología materna, fetal y neonatal que RNAT (n=390). En el análisis univariante el sexo varón y el crecimiento intrauterino restringido (CIR) fueron factores asociados al riesgo de déficit del DPM y en el multivariante solo CIR.
ConclusiónEl riesgo de déficit en el DPM entre PT y RNAT a los 5años parece no evidenciarse con diferencias significativas entre ambos, siendo los valores obtenidos en el ASQ3 ligeramente inferiores en los PT. Sexo varón y CIR parecen influir negativamente en este riesgo.
The term “late preterm” (LPT) was defined in a conference in the United States in 2005 with the aim of optimising the care and outcomes of near-term pregnancies and infants.1 It encompasses infants born between 34 and 37 weeks’ gestation.1,2
The prevalence of LPT birth in singleton pregnancies in developed countries ranged from 3% to 6% in 2010, with these births amounting to 65%–75% of all preterm births.3 Until recently, LPT infants were viewed and managed as full term (FT) infants,1 but numerous reviews in the past 10 years have evinced the physiological immaturity of LPT infants at birth and in subsequent development.4,5 As a result, these infants are at higher risk of complications, not only in the neonatal period2,6 but also in the long term, including, among others, abnormalities in motor, cognitive and behavioural development7–10 that physicians must be aware in order to detect and manage them early, thereby improving developmental outcomes.
The process of cerebral maturation, including neurogenesis and synaptogenesis, takes place in the last weeks of gestation; in addition, the brain of LPT infants weighs 65% of the weight of FT infants and has a lower degree of myelination.11 In LPT infants, the intrauterine development of the brain halts at birth and becomes more vulnerable to factors that may have a negative impact on brain maturation, for instance, admission to the neonatal unit with medical problems,12,13 lack of breastfeeding,14 low weight for gestational age,15 male sex16 and the social and cultural environment of the infant.17
The psychomotor development (PMD) in early childhood is a determinant of behaviour and learning, and the scientific evidence suggests that early detection of problems in PMD allows timely and effective intervention that can change the course of these disorders.18
Paediatric societies recommend universal use of PMD screening tools during early childhood, especially in children with risk factors such as preterm birth or genetic or metabolic disorders.19 Some of these tools are complex, require professionals specifically trained in their use and take too much time. As a result, there is a risk of underdiagnosing mild PMD disorders, as evinced in the literature.20
There are parent questionnaires that can be used to detect PMD problems. One of them is the Ages and Stages Questionnaire (ASQ), a set of questionnaires on child development designed by J. Squires and D. Bricker21 at the University of Oregon that can be completed by parents in 12–18min. In 2009, this tool was revised, leading to publication of the third edition of the Ages and Stages Questionnaire (ASQ3®), which covers ages 2 months to 60 months. The properties of the ASQ3 vary depending on the age range, type of child and the reference used, among others, and a number of studies have found a moderate sensitivity and a high specificity,22 as well as a good correlation with more complex tests.22,23 The ASQ has proven to be valid and consistent for screening of FT children and children with risk factors such as preterm birth.24,25 In addition to the ease of administration, the involvement of parents in the assessment of the children seems to be a positive aspect.26 At present, it is the most widely used parent-completed screen worldwide.23 It has been translated to several languages, including Spanish,23 and has been adapted and validated for use in different countries and regions, including the region of Galicia in Spain.27 The group for surveillance of LPT infants (SEN34-36) of the Sociedad Española de Neonatología (Spanish Society of Neonatology) has recently recommended the use of the ASQ3 at ages 2 years and 4 or 5 years in children born LPT.28
Sample and methodsWe conducted a case–control study comparing PMD at 5 years with a comparative descriptive analysis of perinatal mortality in FT versus LPT infants in a tertiary care hospital. We reviewed the records of all children born LPT in 2010, collecting prenatal and neonatal data.
To compare the population of children born LPT with children born FT, we collected the same data for 2 children born FT of the same sex and born immediately after (no more than 48h after) for each child born LPT. Table 1 presents the collected data.
Prenatal, neonatal and mortality data collected for children born late preterm and full term in the study.
| Maternal problems during gestation | Maternal hypertension | Pre-eclampsia, hypertension, eclampsia, HELLP syndrome |
| Maternal glucose disorder | Gestational diabetes, pregestational diabetes | |
| Other maternal problems | Intrapartum fever, acute infection, chronic infection, autoimmune disorder, cholestasis, maternal drugs | |
| Foetal problems | Intrauterine growth restriction | Diagnosed in foetal ultrasound |
| Acute changes in foetal monitoring | ||
| Abnormalities in placenta or adnexa | Placental abruption, oligoamnios, polyhydramnios | |
| Multiple pregnancy | 2 or more foetuses | |
| Delivery | Spontaneous vaginal, instrumental, caesarean | |
| Resuscitation in delivery room | Need of resuscitation at birth (intermittent positive pressure, intubation, chest compressions and/or epinephrine) | |
| Apgar score | 1-min and 5-min | |
| Sex | ||
| Birth weight | In grams | |
| Admission to neonatal unita | Neonatal ward and/or neonatal intensive care unit (NICU) | |
| Neonatal length of stay | ||
| Neonatal metabolic disorder | Hypoglycaemia | Glucose <45mg/dL |
| Hypocalcaemia | Ionic calcium <0.9mmol/L or total calcium <7mg/dL | |
| Hyperbilirubinaemia | Requiring phototherapy | |
| Neonatal respiratory problems | Respiratory distress syndrome (RDS) | Compatible clinical and radiographic features and/or need of oxygen |
| Transient tachypnoea | Respiratory symptoms in the first 6h of life not meeting criteria for RDS or other disease | |
| Pulmonary air leak | On chest radiograph | |
| Clinically significant neonatal infection | Confirmed vertical sepsis | Excluding mild local infections |
| Confirmed nosocomial sepsis | ||
| Clinical sepsis >72h post birth | ||
| Clinical sepsis <72h post birth | ||
| Congenital infection | ||
| Malformations | Cardiac, renal, gastrointestinal, genital, other (excluding hernias and cryptorchidism) | |
| Neonatal gastrointestinal disorder | Difficulty feeding, gastro-oesophageal reflux, vomiting | |
| Other neonatal disorders | Renal | Acute renal failure… |
| Neurologic | Seizures, intraventricular haemorrhage… | |
| Haematologic | Anaemia, isoimmunization, thrombocytopenia… | |
| Haemodynamic | Hypotension, neonatal pulmonary hypertension, heart rhythm disorders, patent ductus arteriosus… | |
| Death | Up to age 5 years | Causes and date of death |
The criteria for admission in the neonatal ward were: gestational age <35 weeks, birth weight <2200g and any disease requiring treatment or monitoring. For the NICU: need of any form of respiratory or haemodynamic support, central line, severe metabolic, haematologic or endocrine abnormalities, severe neurologic impairment, severe or life-threatening malformations, gestational age <32 weeks and birth weight <1500g.
HELLP, haemolysis, elevated liver enzymes, low platelet count.
To compare the risk of abnormal PMD at 5 years, we sent the ASQ3 questionnaire for age 60 months by conventional mail to the parents of eligible LPT and FT infants, including those that died and with major malformations, central nervous system (CNS) abnormalities and genetic disorders. We sent the ASQ3 questionnaire at 60 months post birth, and the questionnaires had to be completed by 66 months (valid period). We also provided a phone number and email address to participants in case they had any questions.
The ASQ3 assesses 5 domains (communication, gross motor, fine motor, problem solving, and personal-social) with 6 items each and 3 possible answer choices for each item: “yes” (10 points), “sometimes” (5 points) and “not yet” (0 points). We recorded the total overall score of the ASQ3 and the score in each of the domains.
To establish the optimal cut-off point in the total ASQ3 score to discriminate the risk of developmental delays in our sample, we used receiver-operating characteristic (ROC) curve analysis. We found an ideal threshold of 253. This approach has been used before in a similar study.14 We also analysed children with scores more than 2 standard deviations (SDs) below the mean in any of the domains, in accordance with the ASQ3 manual.
In the statistical analysis, we summarised qualitative data as percentages and quantitative data as mean and SD in case of normally distributed variables and as median and interquartile range (IQR) in case of non-normally distributed variables (Shapiro Wilks test, P<.05).
Our study was based on the comparison of independent groups. We compared qualitative variables using the χ2 test or the Fisher exact test if any of the expected cell counts was less than 5 in the 2×2 table. We compared quantitative variables with the Student t test for independent samples or, in case of non-normally distributed variables, the Mann–Whitney U test.
We used univariate logistic regression to analyse the factors associated with developmental deficits in children. We performed a multivariate analysis with the variables corresponding to P-values of up to .1 in the univariate analysis.
We considered differences with a P-value of less than .05 statistically significant. The statistical analysis was performed with the software SPSS version 22.
The study was approved by the research ethics committee of the hospital and we obtained informed consent from the parents of the participants in writing.
ResultsOf the 2565 infants delivered in 2010, 195 (7.6%) were born LPT. The total number of PT births in this period was 276, meaning that 70.6% of all PT births were LPT. A total of 107 LPT infants were male (54.9%).
The FT group included 390 children (2 per each LPT infant).
Table 2 shows the maternal, prenatal and neonatal characteristics of the LPT and FT infants. Among the maternal diseases during pregnancy, we found that hypertension, gestational diabetes and exposure to toxic substances were significantly more frequent in the LPT group. As for foetal problems, we found a significantly higher frequency of intrauterine growth restriction (IUGR), nonreassuring foetal status in the peripartum period and abnormalities of the placenta in LPT infants.
Maternal, prenatal and neonatal data in the full term and late preterm groups and statistically significant differences.
| LPT (n=195) | FT (n=390) | P | OR (95% CI) | |
|---|---|---|---|---|
| Maternal age in years (mean) | 32.6±6.1 | 32.4±5.3 | .53 | |
| Multiple gestation | 28.2% | 1% | <.001 | 38.5 (13.7–108.2) |
| Maternal disease | 40% | 21.5% | <.001 | 2.5 (1.7–3.6) |
| Foetal disease | 28.2% | 13.3% | <.001 | 2.6 (1.7–4) |
| Delivery | ||||
| Normal vaginal | 45.6% | 49.2% | .5 | |
| Caesarean | 41.5% | 21% | <.001 | 3.1 (2.1–4.5) |
| Instrumental | 12.9% | 29.8% | <.001 | 3.3 (2.1–5.3) |
| Resuscitation | 14.1% | 4.9% | <.001 | 3.3 (1.8–6.8) |
| Apgar score (mean) | ||||
| 1-min | 8.5±1.2 | 8.8±0.72 | <.001 | |
| 5-min | 9.65±1.02 | 9.9±0.37 | <.001 | |
| Weight in g (mean) | 2.450±539 | 3.317±447 | <.001 | |
| Neonatal admission | 59.5% | 11.8% | <.001 | 17.1 (7.3–16.9) |
| Neonatal disease | 66.1% | 23.3% | <.001 | 6.5 (4.4–9.5) |
| Metabolic | 59.9% | 10.3% | <.001 | 11.2 (7.3–17.2) |
| Respiratory | 17.4% | 3.6% | <.001 | 5.7 (3–10.9) |
| Gastrointestinal | 15.9% | 1% | <.001 | 17.7 (6.1–51) |
| Infectious | 7.1% | 5.6% | .45 | 1.3 (0.7–2.6) |
| Malformation | 8.7% | 4.6% | <.05 | 2 (1.1–3.9) |
| Haematologic | 6.7% | 3.6% | .09 | 1.9 (0.9–4.2) |
| Haemodynamic | 4.1% | 0.5% | <.01 (Fisher) | 8.4 (1.8–39.9) |
| Neurologic | 2% | 0% | <.05 (Fisher) | – |
| Nephrological | 0.5% | 0.3% | Fisher=.55 | 2 (0.12–32.5) |
CI, confidence interval; FT, full term; LPT, late preterm; OR, odds ratio.
Values of P of less than 0.05 are presented in boldface.
The stay in the neonatal ward of infants admitted during the neonatal period was significantly longer in LPT infants (median, 12.5 days; IQR, 8–17.7) compared to FT infants (median, 5.5 days; IQR, 3–7) (P<.001).
When it came to mortality (excluding major anomalies and malformation syndromes, chromosome disorders and inborn errors of metabolism), we only identified 2 deaths, both in the LPT group (1%): 1 caused by nosocomial sepsis and 1 sudden infant death, both in LPT infants with a history of IUGR. We did not find significant differences between groups (Fisher test, P=.1).
To assess the risk of developmental deficits at age 5 years, we selected 187 of the 195 children born LPT (we excluded 8 children born LPT: 3 that died, 1 with a major gastrointestinal anomaly, 2 with CNS abnormalities and 2 with genetic disorders). We selected 374 children from the FT group.
We received completed ASQ3 questionnaires from 88 participants in the LPT group (47%) and 131 in the FT group (35%). Table 3 presents the characteristics of these children.
Characteristics of children that submitted the ASQ3 and statistically significant differences.
| LPT with ASQ (n=88) | FT with ASQ (n=131) | P | |
|---|---|---|---|
| Birth weight, mean±SD (g) | 2397±476 | 3357±430 | <.001 |
| Gestational age, mean±SD (weeks) | 35.1±0.9 | 39.3±1.2 | <.001 |
| Male | 52.6% | 52.5% | .99 |
| Foetal problems | 36.3% | 12.2% | <.001 |
| Maternal disease | 40.9% | 17.6% | <.001 |
| Twin pregnancy | 28.9% | 1.7% | <.001 |
| Caesarean delivery | 48.7% | 22.4% | <.001 |
| RDR | 15.9% | 3% | <.01 |
| Admission to neonatal ward | 68.4% | 11% | <.001 |
| Admission to NICU | 31.8% | 3.8% | <.001 |
| Neonatal problems | 73.8% | 19.8% | <.001 |
| Mother with university education | 49.4% | 66.1% | .02 |
| Maternal age, mean±SD (years) | 35.1±5.2 | 33.4±4.6 | .02 |
| Breastfeeding | 47.3% | 71.5% | <.01 |
FT, full term; LPT, late preterm; NICU, neonatal intensive care unit; RDR, resuscitation in the delivery room; SD, standard deviation.
Values of P of less than 0.05 are presented in boldface.
When we compared the LPT infants for which we received the completed ASQ3 versus those for which we did not, we did not find statistically significant differences in the perinatal variables that may have had an impact on developmental outcomes except for the overall frequency of foetal problems (although we did not find a significant difference in the history of IUGR, which is the most common foetal problem). We did not find any differences in these variables when it came to FT infants (Table 4).
Characteristics and history of LPT infants and FT infants for which we did and did not obtain a completed ASQ3, comparison and statistical significance.
| LPT with ASQ (n=88) | LPT without ASQ (n=99) | P | FT with ASQ (n=131) | FT without ASQ (n=243) | P | |
|---|---|---|---|---|---|---|
| Weight (g), mean±SD | 2.397±476 | 2.516±600 | .13 | 3.357±430 | 3.299±439 | .27 |
| Gestational age (weeks), mean±SD | 35.1±0.9 | 35.1±0.8 | .35 | 39.3±1.2 | 39.4±1.2 | .51 |
| Male sex | 52.6% | 55.8% | .66 | 52.5% | 52.3% | .94 |
| Multiple gestation | 28.9% | 28.8% | .98 | 1.5% | 0.4% | .23 (Fisher) |
| Maternal disease | 43.4% | 36% | .31 | 19.8% | 21.7% | .64 |
| Foetal disease | 35.5% | 20.7% | .03 | 12.8% | 12.1% | .83 |
| IUGR | 14.5% | 6.3% | .08 | 0.8% | 1.2% | 1 (Fisher) |
| RDR | 18.4% | 11.8% | .19 | 2.3% | 4.8% | .4 (Fisher) |
| Caesarean | 48.7% | 36% | .08 | 22% | 16.2% | .17 |
| NICU admission | 30.3% | 21.6% | .08 | 3.4% | 1.6% | .27 (Fisher) |
FT, full term; IUGR, intrauterine growth restriction; LPT, late preterm; NICU, neonatal intensive care unit; RDR, resuscitation in delivery room; SD, standard deviation.
Values of P of less than 0.05 are presented in boldface.
The mean total ASQ3 score and domain scores were lower in the LPT group, although we did not find statistically significant differences in any of them (Table 5).
ASQ3 in children born late preterm versus full term. Domain scores, total score and statistical significance.
| LPT (n=88) | FT (n=131) | P | |
|---|---|---|---|
| Communications | |||
| Mean±SD | 55.8±8 | 56.6±4.8 | .57 |
| Median (IQR) | 55 (55–60) | 60 (55–60) | |
| Gross motor | |||
| Mean±SD | 52.9±10.7 | 55.5±6.2 | .1 |
| Median (IQR) | 55 (50–60) | 60 (50–60) | |
| Fine motor | |||
| Mean±SD | 55.3±7.9 | 56.2±5.6 | .19 |
| Median (IQR) | 55 (55–60) | 60 (55–60) | |
| Problem solving | |||
| Mean±SD | 58.3±2.6 | 59.5±1.6 | .12 |
| Median (IQR) | 60 (60–60) | 60 (60–60) | |
| Personal-social | |||
| Mean±SD | 55±7.7 | 56±5 | .79 |
| Median (IQR) | 55 (55–60) | 55 (55–60) | |
| Total | |||
| Mean±SD | 277.3±30.2 | 283.9±14.6 | .13 |
| Median (IQR) | 285 (275–290) | 285 (275–295) | |
FT, full term; IQR, interquartile range; LPT, late preterm; SD, standard deviation.
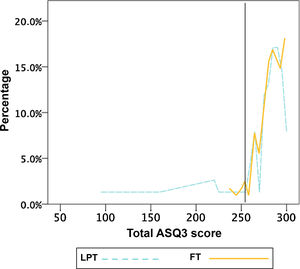
When we graphed the percentage distribution of the total ASQ3 scores (Fig. 1), we found that both groups had an equivalent distribution starting from 253 points, and that below this point there was a higher proportion of LPT infants with lower scores. The ROC curve analysis found a sensitivity greater than 81% for this cut-off point. In addition, this threshold was near the value corresponding to 2 SDs below the mean of the reference FT population (254.7 points). The Kolmogorov–Smirnov test did not reveal any differences in the percentage distribution between the two groups above the threshold (P=.74).
We identified 11 children at risk of delayed PMD (≤253 points). Seven were in the LPT group (7.9%) and 4 in the FT group (3%), with a higher percentage in the LPT group, although not significantly so (Fisher test P=.1; odds ratio [OR], 2.7 [0.8–9.7]). Of these 11 children, 8 had at least 1 affected (score more than 2 SD below mean, as established in the manual). Of the 7 children born LPT, 6 had an abnormal score in some domain, chiefly the gross or fine motor skills domain, and 3 had abnormal scores in more than one domain. We also found deficits in the personal-social and communication domains that were not observed in the FT group. Of the 4 FT children, 2 had an affected domain (gross motor in 1 and fine motor in 1). We did not find abnormal scores in the problem solving domain in any of the children.
The 7 LPT children at risk of developmental delay were male: 3 had a history of hypoglycaemia in the neonatal period (as the only relevant disease), 3 had a history of IUGR, and only 1 required admission to the NICU due to a history of IUGR with a birth weight of 1500g.
Of the 4 FT children at risk of developmental delay, half were male: 3 had a relevant neonatal history, and none had required admission to the NICU: 2 had jaundice (1 of them also had anaemia) and 1 had transient respiratory distress. We did not find a relevant history of foetal or maternal disease in any of these children.
In the univariate analysis, which included potential risk factors for developmental abnormalities, the only variables that were significant were sex (male) and IUGR (the main disease in the foetal period). The P-value for LPT birth was near the threshold of significance. In the multivariate analysis (which included variables with a P≤.1 in the univariate analysis) the only variable that continued to be significant was IUGR (Table 6).
Univariate and multivariate* regression analysis (*including variables with P≤.1 in the univariate analysis) of potential risk factors for abnormal psychomotor development based on the ASQ3.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | |
| Late preterm birth | .1 | 2.7 (0.8–9.7) | .3 | 1.9 (0.5–7.6) |
| Sex (male) | .04 | 4.4 (1.1–19.7) | .08 | 4.1 (0.8–19.9) |
| Twin gestation | .99 | – | ||
| Resuscitation | .9 | 1.1 (0.1–9.3) | ||
| Caesarean delivery | .38 | 1.7 (0.5–5.9) | ||
| Foetal problems | .06 | 3.2 (0.9–10.9) | ||
| IUGR | <.01 | 7.4 (1.7–32.3) | .04 | 5.3 (1.1–26.4) |
| Maternal disease | .98 | 1.1 (0.3–3.9) | ||
| Admission to NICU | .26 | 2.2 (0.6–8.8) | ||
| Respiratory problems | .47 | 1.8 (0.4–8.8) | ||
| Metabolic disorders | .23 | 2.1 (0.6–7.1) | ||
| Hypoglycaemia | .28 | 2.8 (0.4–18) | ||
| Jaundice | .45 | 1.6 (0.5–5.8) | ||
| Gastrointestinal disease | .23 | 2.7 (0.5–13.4) | ||
| Infectious disease | .99 | – | ||
| Mother without university education | .27 | 2 (0.6–6.7) | ||
| Absence of BF | .88 | 1.1 (0.3–4) | ||
| Maternal age | .97 | 0.97 (0.9–1.1) | ||
BF, breastfeeding; CI, confidence interval; IUGR, intrauterine growth restriction; NICU, neonatal intensive care unit; OR, odds ratio.
Values of P of less than .1 (univariate) and .05 (multivariate) are presented in boldface.
When we combined the factors of LPT birth and male sex and of LPT birth and IUGR, we found significantly increases in risk (P<.01; OR, 7.1 [2–25.5] and P<.01; OR, 8.3 [1.9–36.6], respectively).
DiscussionIn our study, the frequency of LPT birth was similar to the frequency reported in the recent literature in developed countries.3 A study in 34 Spanish hospitals29 conducted between 2011 and 2016 found a proportion of LPT birth of 5.9%, somewhat lower compared to the one in our study (7.6%). Although the morbidity experienced by LPT infants in the neonatal period is not as significant as the morbidity in more premature infants, the frequency of LPT birth is large enough for it to give rise to significant health care use and have a substantial economic impact in neonatal care settings.5
The increase in LPT birth observed in recent years has been attributed to several reasons, including increased use of prenatal care (with more frequent detection of foetal and/or maternal problems and voluntary termination of pregnancy) and obstetric interventions, an increased frequency of multiple pregnancy, use of assisted reproductive techniques, greater maternal age and increased frequency of complications.1,2 In our study, we found greater proportions of multiple pregnancy and maternal and foetal problems in the LPT group compared to the FT group, which in many instances were the cause of preterm birth and may have been associated with subsequent morbidity.
Decreased growth and physiological immaturity in LPT newborns may lead to complications in both the short and the long term.4,5,30 In our study, we found a higher neonatal morbidity in this group compared to FT infants, which was consistent with the previous literature.4
Although in our study there were only deaths in the LPT group, this difference in relation to the FT group was not statistically significant. We may have found a significant difference in mortality with a larger sample, as described in some literature reviews.5,30
As for PMD in children born LPT, literature reviews suggest an increase in motor, cognitive and behavioural problems compared to children born at term.7–10 McGowan et al.9 published a systematic review of 10 studies that assessed PMD in children born LPT between ages 1 and 10 years. Although variable measurement and the exclusion criteria for LPT infants varied between studies, the review found a greater frequency of developmental abnormalities (defined as cerebral palsy, global development delay, intellectual disability or language disorders). Another review including assessments between ages 2 and 36 years found a small but significant increase of adverse cognitive and educational outcomes and concluded that children born LPT could benefit from more rigorous monitoring of PMD and academic performance.10 The differences in development between children born LPT versus FT seem more marked in the early years of life, but are not as large at later ages, and some studies have not found significant differences at 8–11 years31 or 4–15 years (in “healthy” children born LPT).32 This “developmental catchup” in the first years of life may suggest that some of the detected abnormalities are temporary and that performance in some areas may improve with schooling.33
The frequent time constraints in paediatric visits and the complexity of the “professional” tools used in the evaluation of PMD may result in delayed diagnosis or underdiagnosis in children at risk, and the ASQ appears to be a very useful alternative.27
A study conducted in Spain that administered the ASQ in children born LPT versus FT at ages 24 and 48 months found that the presence of abnormal scores in at least 2 domains at 24 months was significantly correlated to the risk of developmental problems at 48 months, especially in children born LPT. Furthermore, the authors only identified abnormal scores in 2 or more domains in the LPT group.25
Demestre et al.14 reported a lower mean total score and lower domain scores in children born LPT compared to children born FT at age 4 years, but without statistically significant differences, as was the case in our study. They did detect a significantly higher risk of developmental problems in the LPT group (total ASQ≤251 points). In our study, the proportion of children at risk of PMD problems was more than twice greater in the LPT group compared to the FT group, although this difference was not statistically significant. To find significant differences, a larger sample may be needed, or perhaps for these differences to decrease at age 5 years. Motor domains were most affected in both groups, but we found a higher frequency of abnormalities in the LPT groups, with several children in this group having abnormal scores in more than one domain.
We ought to mention that Hornman et al.33 found patterns of persistence, emergence or disappearance in the risk of developmental problems, which seemed to vary based on age, the degree of prematurity and intervention. They found similar patterns in children born FT and moderately PT (32–35 weeks’ gestation) between ages 4 and 5 years, in contrast with children born PT before 32 weeks’ gestation, who experienced more persistent and emergent problems. The significant difference observed at age 4 years between children born moderately PT, with poorer total and communication domain scores in the ASQ compared to children born FT, disappeared at 5 years. On the other hand, the authors did find a worsening in motor skills and improvement in communication, which suggested that the combination of reduced physical activity at home limited time spent in exercise in school could account for the emerging motor problems in children born preterm after school entry.
Kerstjens et al.34 found that other factors are at play in developmental abnormalities in addition to LPT birth. Chief among them were male sex, caesarean delivery, preeclampsia, low birth weight for gestational age, twin gestation, admission to the NICU, low socioeconomic status and absence of breastfeeding.12,17,34–36 Admission to the neonatal ward is affected by protocols that differ between hospitals, and therefore it seems more appropriate to use as a variable the admission to the neonatal intensive care unit (NICU). As occurred in our study, Kerstjens et al.13 did not find less favourable outcomes at 43–49 months in infants that required NICU admission compared to those that did not based on the ASQ results, although the P-value approached the threshold of significance. In contrast, Baron et al.37 did find a higher frequency of cognitive impairment in LPT infants that required admission to the NICU (Differential Ability Scales, 2nd edition, preschool children). Neonatal problems commonly found in the NICU, such as hypoxia, metabolic disorders and sepsis, could have an impact on neurologic impairment in children born LPT.8 In our study, we found a history of hypoglycaemia and hyperbilirubinaemia in the neonatal period in some of the children at risk of abnormal PMD, although we did not find a significant association as other studies did.13,38 Other variables found to be significantly associated in previous studies, such as absence of breastfeeding,14,17 caesarean delivery,35 maternal disease during pregnancy, such as preeclampsia,17 resuscitation in the delivery room,39 parental educational attainment,16 young maternal age40 or twin gestation,36 were not found to be significant factors in our study. At any rate, the results of these studies are heterogeneous and it appears that these variables do not have a consistent impact on PMD.
Our study did find an increased risk of developmental abnormalities in association with male sex and, above all, the history of IUGR, which would compound the risk associated with LPT birth. These two variables have been frequently identified as risk factors in other studies, although some of them use the variable “small for gestational age” instead, which in most cases corresponds to a history of IUGR.12,15,16,34,37
It is possible that many of the developmental delays detected in LPT children correspond to subtle abnormalities that could be managed simply with observation and would resolve spontaneously without intervention. But it seems appropriate to use this questionnaire to monitor development in this risk group and identify these deficits, as recommended by the SEN34-36, the working group of specialists in Spain.28 Furthermore, the age of 4–5 years is the last interval for the detection of developmental abnormalities that could benefit from intervention.
We ought to mention that when using the ASQ, assessing the risk of deficits in specific domains of PMD decreases the sensitivity and increases the specificity of the result, which is why some authors choose, as we did in the present study, to use the total score. Indeed, in our study we identified a greater number of children with possible developmental deficits based on the total score of the ASQ3, and the group of children identified with this criterion included all the children with abnormal results in individual domains, which denotes a greater sensitivity a priori of the criterion selected in this study.
One of the limitations of the study is the small number of children for which we received a completed ASQ3. There is a risk of response bias, as it is possible that only parents concerned about developmental problems in their children responded to the survey. In addition, we did not collect data on potential therapy received in early intervention programmes, which could have an impact on development.
ConclusionLate preterm infants constitute a large group, have more perinatal complications than FT infants and require specific monitoring of PMD in the first years of life. Male sex and a history of foetal problems, such as IUGR, seem to further increase the risk already associated with LPT birth. The findings of our study suggest that although differences with FT infants seem to level out, as evinced by the lack of significant differences in the ASQ3, there is a mild negative impact on PMD in LPT children, as we found slightly lower values in the questionnaire and a greater probability of developmental abnormalities in gross and fine motor skills. Subtle abnormalities are difficult to detect, and therefore the ASQ may be a useful supplemental tool in the clinical evaluation that is also easy to use, involves parents and helps identify problem areas with the aim of addressing them and minimising long-term sequelae.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Morales-Luengo F, Salamanca-Zarzuela B, Fernández Colomer B. Desarrollo psicomotor en prematuros tardíos a los cinco años de edad: comparación con recién nacidos a término mediante ASQ3®. An Pediatr (Barc). 2021;94:301–310.