Patients with moderate-severe cerebral palsy (CP) require the support of their caregivers to carry out the Activities of Daily Living (ADLs). Objectives: to describe the comorbidities, need for care in children with CP and to analyse the influence of the degree of motor involvement, nutritional status and other neurological disorders.
MethodsCross-sectional and observational study. Patients with CP degrees III–IV–V according to the Gross Motor Function Classification System (GMFCS) have been studied. A record of comorbidities has been made and body composition has been studied using anthropometry and bioimpedance. In addition, a caregiver burden survey on ADLs has been carried out (10 items on the different actions: hygiene, clothing, transfers, sleeping and feeding). Which variables have the greatest influence on the perception of difficulty in performing ADLs have been studied.
Results69 patients (50.7% women, mean age 10.46 ± 0.4 years) were analysed, with GMFCS grades: grade III 36.2% (N = 25), grade IV 29.0% (N = 20), grade V 34.8% (N = 24). A relationship was found between the caregiver burden score and GMFCS grade (p = 0.003) and intellectual disability (DI) (p < 0.001). However, regardless of the degree of GMFCS and DI, there is greater difficulty in performing ADLs in relation to lower values in weight (Z-score) (p = 0.028), fat mass (kilograms) (p = 0.035), fat mass (%) (p = 0.094), body mass index (Z-score) (p = 0.086).
ConclusionsIn addition to the degree of clinical impairment, nutritional status is a factor that influences the caregiver's difficulty in performing the ADLs in CP patients on which we can act to improve this problem.
Los pacientes con parálisis cerebral (PC) moderada-grave precisan de sus cuidadores para realizar las Actividades Básicas de la Vida Diaria (ABVD). Objetivos: describir la presencia de comorbilidades, la percepción de dificultad de los cuidadores para realizar las ABVD y analizar la influencia del grado de afectación motora, el estado nutricional y otras alteraciones neurológicas.
Material y métodosEstudio transversal y observacional. Se estudió a pacientes con PC grados III–IV–V según el Sistema de Clasificación de la Función Motora Gruesa (GMFCS). Se realizó un registro de comorbilidades y se estudió la composición corporal mediante antropometría y bioimpedanciometría. Además, se realizó una encuesta de carga del cuidador sobre ABVD (10 ítems que exploran las diferentes actuaciones: higiene, vestir, transferencias, sueño y alimentación). Se estudió qué variables influyen más en la percepción de dificultad para realizar las ABVD.
ResultadosMuestra: 69 pacientes (50,7% mujeres, edad media 10,46 ± 0,4 años), con grados GMFCS: grado III 36,2% (N = 25), grado IV 29,0% (N = 20), grado V 34,8% (N = 24). Se halló relación entre la puntuación de carga del cuidador y grado GMFCS (p = 0,003) y discapacidad intelectual (DI) (p < 0,001). Además, independientemente del grado de GMFCS y DI, existe mayor dificultad para realizar las ABVD en relación con valores más bajos en peso (Z-score) (p = 0,028), masa grasa (kilogramos) (p = 0,035), masa grasa (%) (p = 0,094), índice de masa corporal (Z-score) (p = 0,086).
ConclusionesAdemás del grado de afectación motora y la discapacidad intelectual, el estado nutricional es uno de los factores modificables que influyen en la dificultad del cuidador para realizar las ABVD en los pacientes con PC.
Cerebral palsy is the most frequent cause of motor impairment in the paediatric population. The term refers to a disorder of motor development and posture attributed to a non-progressive lesion to developing brain during foetal life or the early years of life.
A study of the surveillance of cerebral palsy in Europe1 established the frequency of cerebral palsy at 2–3 cases per 1000 live births. This study recommends a minimum age of 3 years for diagnosis of cerebral palsy and considers 5 years the optimal age.
Patients with cerebral palsy often have multiple comorbidities with an impact on the mental and physical health of the patient as well as the caregiver that require the investment of substantial time and resources.2 Nutritional complications are among the most frequent comorbidities, and they can be acted on directly.3
In this regard, it is important to try to maintain an optimal nutritional status in these patients to allow them to develop their motor, cognitive, communication and social skills, thus contributing to global neurodevelopment2,4 in addition to preventing undernutrition and diseases associated with nutritional deficiencies.5 Although it seems evident that greater motor impairment will increase the burden of caregivers of patients with cerebral palsy, we do not know the impact of nutritional status on caregiving.6
The objectives of our study were to establish the prevalence of comorbidities in a population of patients with moderate-to-severe cerebral palsy and assess the difficulty perceived by caregivers in carrying out activities of daily living (ADL) with these patients as well as its potential association with nutritional status, the degree of motor impairment and other neurologic abnormalities.
Material and methodsWe conducted a cross-sectional, observational, descriptive and analytical study. The study universe comprised patients aged 4–15 years with moderate-to-severe spastic cerebral palsy managed in the paediatric neurology unit of a tertiary care hospital.
We included patients classified as level III, IV or V in the Gross Motor Function Classification System (GMFCS) developed by Palisano7 and modified according to the International Classification of Functioning, Disability and Health of the World Health Organization, which classifies patients into groups according to the level of motor impairment. We excluded patients who themselves or whose parents refused to participate, patients outside the established age range or patients that did not meet the criteria for diagnosis of cerebral palsy.
The study was approved before its initiation by the Regional Ethics Committee (CEICA; PI16/039). We obtained signed informed consent from all the patients or their parents or legal guardians.
After verifying that the patients were eligible for inclusion, we contacted them or their legal guardians to schedule a visit to the clinic, where we performed a clinical interview and an examination to collect data on the clinical condition of the patient, anthropometric measurements and dietary habits. We collected data on neurologic variables (visual impairment, hearing loss, intellectual disability etc.) from the paediatric neurology records of the patients with confirmation by the paediatric neurologist.
We used a short questionnaire to identify problems in nutrition. It comprised 4 items (positive result, ≥2 items): Do meals take more than 30 min? Are meals a stressful time for the caregiver or child? Does the child exhibit adequate weight gain? Does the child exhibit respiratory symptoms during or after meals? We classified as having dysphagia patients with 2 or more positive items in the questionnaire (and whose problems were associated with swallowing/eating), patients with compatible findings in the swallowing study and patients that had a gastrostomy due to feeding problems related to swallowing.
We interviewed caregivers to assess the difficulty they perceived helping the child carry out ADL. To this end, we used a questionnaire in Spanish validated for use in parents of patients with moderate-to-severe cerebral palsy.8 The questionnaire is composed of 10 items (on personal hygiene, transferring, feeding, dressing and sleep) scored between 0 and 3 points (easy-very difficult) to measure perceived difficulties (maximum possible difficulty, 30 points).
The diagnosis of constipation was based on the Roma IV clinical criteria for diagnosis of constipation in children9 and the Bristol stool chart.10 The group of patients with gastro-oesophageal reflux included those with a positive pH test and those with clear manifestations of reflux (frequent regurgitation and vomiting).
The anthropometric evaluation included measurement of the weight and height, with the latter estimated using the knee height equation proposed by Stevenson11: Height = (knee height × 2.69) + 24.2 cm. The data were collected with a scale (weight) and callipers (knee height). We calculated the z-scores for the anthropometric measurements using the growth charts published by Carrascosa et al. in 2010 as Reference 12, in adherence with the recommendation of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) of 2017 to use growth charts from the reference population. We calculated the body mass index (BMI) and the weight-for-height (%WFH or Waterlow classification) to categorize the nutritional status of the patient based on weight: %WFH = actual weight/expected weight for height (50th percentile) × 100.
We assessed the body composition of patients by bioimpedance analysis (BIA) with an Akern BIA-101 Anniversary analyser. This method provides data on the total body water (extracellular and intracellular), fat mass, body cell mass (total mass of living, functioning and metabolically active cells) and fat-free mass (lean soft tissue and bone).13,14 This is achieved by applying an imperceptible alternating current through electrodes attached to the skin that detect resistance (opposition to the flow of current) and reactance (delay in conduction caused by cell membranes, tissue interfaces and non-ionic substances).13 The BIA protocol used has been described in greater detail in a previous publication.15 The same article explained how we adjusted the fat-free mass of patients for height, age and sex to obtain the “percent of the expected fat-free mass” (optimal fat-free mass).
The statistical analysis was performed with the software SPSS Statistics, version 21.0. We conducted a descriptive analysis, expressing results as mean ± standard deviation and 95% confidence interval (CI) for quantitative variables or as frequencies for qualitative variables. We assessed the normality of the distribution by means of the Kolmogorov-Smirnov and Shapiro-Wilk tests. For hypothesis testing, we used the χ2 and Fisher exact tests (qualitative variables), the Student t-test (parametric quantitative and dichotomous qualitative variables), Mann–Whitney U test (nonparametric quantitative and dichotomous qualitative variables), analysis of variance (ANOVA) with the Bonferroni correction (parametric quantitative and non-dichotomous qualitative variables) and Kruskal–Wallis test (nonparametric quantitative and non-dichotomous qualitative variables). Lastly, we analysed the correlation between the score in the caregiver questionnaire and the different anthropometric measurements and body composition variables using the Pearson r (normal distribution) and Spearman rho (nonparametric variables). As described in a previous article, in this sample, the anthropometric measurements and body composition of the patients varied based on the degree of motor impairment as assessed by the GMFCS,15 so we fitted a multivariate statistical model including the different anthropometric and body composition variables and variables of motor and neurologic impairment that had exhibited a significant association with the score in the caregiver questionnaire (GMFCS level and intellectual disability) to determine the actual weight of these nutritional variables.
ResultsA total of 82 patients met the inclusion criteria, but 13 refused to participate or could not be located, so the final sample included 69 patients, corresponding to a participation rate of 84.2%. Of this total, 50.7% (n = 35) were female. The distribution of the sample based on the GMFCS was: level III, 36.2% (n = 25); level IV, 29.0% (n = 20) and level V, 34.8% (n = 24). The mean age was 10.46 ± 0.4 years (level III, 10.83 ± 0.7 years; level IV, 10.89 ± 0.8 years; level V, 9.73 ± 0.7 years; P = .380).
Table 1 presents the prevalence of the different comorbidities identified in the sample. We found a high prevalence of comorbidities in patients with moderate-to-severe cerebral palsy; the most frequent were intellectual disability, incontinence, visual impairment, dysphagia, drooling and constipation. We also found an increased frequency of intellectual disability, absence of speech, drooling and poor bowel/bladder control in patients with a higher degree of motor impairment.
Prevalence of comorbidities in patients with cerebral palsy by GMFCS level.
| Total % (n) | Level III (%) | Level IV (%) | Level V (%) | p | |
|---|---|---|---|---|---|
| Intellectual disability | 78.3 (54) | 44 | 95 | 100 | <.001 |
| Absence of speech | 4.6 (28) | 0 | 35 | 87.5 | <.001 |
| Incontinence | 74 (51) | 24 | 70 | 95.8 | <.001 |
| Visual impairment | 59.4 (41) | 56 | 65 | 58.3 | .822 |
| Hearing loss | 11.6 (8) | 12 | 10 | 12.5 | – |
| Seizures in past 12 months (Epilepsy) | 17.4 (12) | 16 | 10 | 17.4 | .415 |
| ADHD | 8.7 (6) | 24 | 0 | 0 | – |
| ASD | 5.8 (4) | 0 | 20 | 0 | – |
| Drooling | 44.9 (31) | 20 | 45 | 7.2 | .002 |
| Dysphagia | 56.5 (39) | 36 | 50 | 83.3 | .003 |
| Gastro-oesophageal reflux | 24.6 (17) | 8 | 10 | 54.2 | <.001 |
| Constipation | 66.7 (46) | 60 | 60 | 79.2 | .274 |
ADHD, attention-deficit hyperactivity disorder; ASD, autism spectrum disorder.
The distribution of patients by type of schooling was as follows: GMFCS III (32% in regular education, 36% in regular education with curricular adaptation and 32% in special education), GMFCS IV (5% regular, 5% with curricular adaptation and 90% special education), GMFCS V (100% in special education) (P < .001).
The mean hours per week that patients spent in occupational/physical therapy was 3.83 ± 1.64 h of therapy delivered by a professional and 1.83 ± 2.59 h delivered by the caregiver at home for a total of 5.66 ± 3.12 h, without significant differences based on the GMFCS. Sixty-four percent of patients in level III, 95% of patients in level IV and 100% in level V received speech therapy regularly (P < .001).
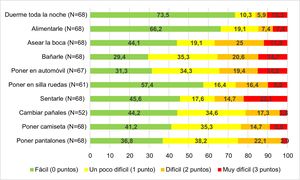
We administered a questionnaire to measure the difficulty perceived by parents/legal guardians in ADL, save for 1 case in which a language barrier would hinder the interpretation of the results. The mean score in the caregiver questionnaire was 8.97 ± 5.90 points: 5.79 ± 1.02 for level III; 9.45 ± 1.44 for level IV and11.75 ± 0.98 for level V (P = .003). The score was 9.17 ± 0.66 for caregivers of patients with intellectual disability compared to 2.93 ± 0.67 for caregivers of patients without intellectual disability (P < .001). We did not find an association between the score in the caregiver questionnaire and hearing loss, visual impairment of absence of speech in the patient. Fig. 1 presents the difficulty perceived by parents in the performance of different ADL with their children.
Table 2 presents the correlations between the score in the caregiver questionnaire on the difficulties experienced in ADLs and the different anthropometric and body composition variables under study. We found a significant inverse correlation between most anthropometric and body composition variables and the caregiver ADL difficulty score, both in the raw measurements and in the measurements adjusted for age and sex (weight z-score, BMI, %WFH). However, when we adjusted this correlation to the level of the patient in the GMFCS and to the presence of intellectual disability, the variables that continued to show a significant association were the weight z-score and the lean mass, while the BMI z-score was near the threshold of significance.
Correlation between anthropometric and body composition variables and the score obtained in the questionnaire assessing the level of difficulty experienced by caregivers in activities of daily living.
| Anthropometric measurements | |||
|---|---|---|---|
| r | P* | P** | |
| Height (cm) | −0.187 | .130 | .501 |
| Height (z) | −0.280 | .021 | .219 |
| Weight (kg) | −0.283 | .019 | .128 |
| Weight (z) | −0.435 | <.001 | .028 |
| BMI (kg/m2) | −0.361 | .003 | .124 |
| BMI (z) | −0.359 | .003 | .086 |
| %WFH | −0.334 | .005 | .186 |
| Bioimpedance analysis-body composition | |||
|---|---|---|---|
| Fat mass (kg) | −0.354 | .003 | .035 |
| Fat mass (%) | −0.279 | .021 | .094 |
| Fat-free mass (kg) | −0.247 | .042 | .270 |
| Fat-free mass (%) | 0.311 | .010 | .059 |
| Fat-free mass (% expected) | −0.151 | .219 | .830 |
r: correlation between anthropometric and body composition variables and the score obtained in the caregiver ADL difficulty questionnaire.
Patients with moderate-to-severe cerebral palsy frequently have comorbidities, the prevalence of which increases with the degree of motor impairment,16 as observed in the sample under study. All these comorbidities entail different care measures and therapies that require coordination of care between primary care and specialized care, although the main burden falls to the caregiver, who needs time, support and technical skills to perform these tasks.
The figure of the caregiver is currently attracting interest and it is likely to become more prominent in the future. Different studies have found an association between the presence of comorbidities and their severity with caregiver quality of life, and therefore the appropriate diagnosis, management and treatment of these comorbidities can help improve this situation.17,18 Thus, we should devote increasing attention to the caregiver as an essential figure that may develop health problems, both physical and mental, derived from caring for the cerebral palsy patient every day, and deserving of individualised care themselves.19 In these patients, there may be many nonmodifiable factors at play that impact the quality of life of caregivers, with motor impairments and behavioural manifestations having the most significant impact.20
As expected, the difficulty experienced by caregivers of patients with cerebral palsy in ADLs, increases with the level in the GMFCS and with the diagnosis of intellectual disability. However, it is interesting that variables used to assess weight gain and nutritional status, independently of patient age or sex, are significantly and negatively associated with the score in the caregiver ADL difficulties questionnaire. Higher weight z-scores, BMI z-scores and percentages of body fat were associated with a decrease in the difficulty perceived by caregivers in relation to ADLs. Patients with cerebral palsy have been found to have more nutritional abnormalities in correlation with the GMFCS level,15 and, at the same time, the GMFCS level and intellectual disability are associated with difficulties in ADLs. Therefore, we had to analyse the results of our study regarding the association between nutritional status and difficulties in ADLs trying to correct as much as possible for the influence of the severity of cerebral palsy. This is important because, contrary to what would be expected, patients weighing more does not seem to pose a problem in these ADLs for the caregivers. In fact, a better nutritional status, independently of the degree of motor impairment and age of the patient, seemed to facilitate these tasks. Motor impairment and intellectual disability are 2 of the factors that have the most impact on the burden of caregivers, but they are hardly modifiable, whereas the nutritional status of patients with cerebral palsy is a factor that can be improved. Given all of the above, the findings of our study support the recommendations of the ESPGHAN regarding the need to routinely provide nutritional support to paediatric patients with cerebral palsy.3
Another important factor on which it is possible to intervene is sleep. Some studies have found evidence of a higher prevalence of sleep disorders in patients with cerebral palsy, which seems to increase with the GMFCS level and can also have an impact on the quality of life of caregivers of patients with moderate-to-severe cerebral palsy.21 In the sample under study, there seemed to be difficulties in caregiving associated with night-time sleep in more than 25% of cases, although this was not associated with nutritional status. Some of the sleep-related complications, such as obstructive apnoea, insomnia and abnormal sleep patterns, such as multiple awakenings or short sleep duration, can and must be addressed given the considerable impact they have on the quality of life of both patient and caregiver.17,21
Dysphagia is another modifiable factor that is very prevalent in cerebral palsy and has an impact on the quality of life of patients and caregivers, and in both our sample and previous case series, the frequency and severity of dysphagia tends to increase with the level of motor impairment.22 In these patients, dysphagia is associated with the presence of nutritional problems, so a comprehensive approach must be used to manage both dysphagia and nutrition problems.16,23 Fortunately, different options are available to address this problem with the use of thickeners, improving textures, support through speech therapy and performance of gastrostomy if necessary.24 One third of patients with level V impairment in our study had a gastrostomy, although we found no differences in nutritional status between the patients with and without a gastrostomy. This could be due to delay placement of a gastrostomy tube or the higher level of motor and neurologic impairment in these patients.15
Some therapies, such as occupational therapy, physical therapy and speech therapy, are very beneficial for the development and wellbeing of patients with cerebral palsy,25 although it must be taken into account that they are time consuming and require regular attendance, thus impinging on the schedule of caregivers and involving multiple trips. Although their usefulness in the management of different aspects related to neuromotor functioning is clear,26 our study did not find evidence of an association between the number of hours devoted to these therapies and relevant differences in the scores of the caregiver difficulty in ADLs questionnaire. Another factor that seems to have a beneficial impact on quality of life of patients and caregivers was schooling adapted to the needs of the patient.18 In our study, all the patients attended school.
Although we did not evaluate it in this study, the economic burden is also significant and is another aspect associated with the level of motor and neurologic impairment of patients.27 A multicentre study conducted in Europe established that neither an optimal financial situation nor the availability of adequate equipment had an effect on the prevention of stress and other psychological problems in caregivers.20 However, a review on the quality of life of caregivers of patients with cerebral palsy concluded that the high prevalence of changes in quality of life and physical and mental health could improve by increasing self-efficacy,28 that is, the capacity and confidence of caregivers in their own competence to perform ADLs correctly.
One of the main limitations of the study was the lack of a larger sample to achieve greater statistical power and be able to do more subgroup analyses. Nevertheless, the sample consisted of a majority of the patients managed in a cerebral palsy reference unit. In future studies, it would be useful to increase the number of patients per GMFCS level and to assess the potential impact of adequate nutritional intervention in the score of the caregiver ADL difficulty questionnaire.
In conclusion, it is reasonable to state that cerebral palsy is a chronic disease associated with multiple comorbidities that require multidisciplinary management and for the direct caregivers of the patient to be adequately trained and educated. To the extent possible, health care professionals should facilitate the work of caregivers to improve their quality of life and patient outcomes. The prevalence of comorbidities, the need for care and the difficulties in ADLs perceived by caregivers increase with the level of motor impairment and intellectual disability. In addition, and independently, nutritional status is one of the modifiable factors that influence the difficulties in ADLs experienced by caregivers of patients with cerebral palsy.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martínez de Zabarte Fernández JM, Ros Arnal I, Peña Segura JL, García Romero R, Rodríguez Martínez G. Carga del cuidador del paciente con parálisis cerebral moderada-grave: ¿influye el estado nutricional? An Pediatr (Barc). 2021;94:311–317.