Models for estimating the risk of sudden cardiac death (SCD) in pediatric hypertrophic cardiomyopathy (HCM) used in our setting do not consider some parameters of routine clinical practice. The objective was to identify non-classical risk factors and evaluate their prognostic value.
Patients and methodsRetrospective observational study, including patients with isolated HCM 0-18 years old, evaluating clinical, genetic, and imaging variables. The risk of SCD or major arrhythmic cardiac events (MACEs) was estimated according to the three most widely used European models (HCM Risk-SCD, European Society of Cardiology [ESC] algorithm, and HCM Risk-Kids), analyzing their predictive capacity by adding genotyping and advanced cardiac imaging parameters.
ResultsThe sample included 77 patients followed up for 5.25 years. Ten (13%) experienced a MACE. We found that MACE was significantly associated with myocardial deformation and positive genotype status, and associated, although not significantly, to late gadolinium enhancement (LGE) in cardiac MRI (P = .062). Events were more frequent (hazard ratio = 18.5; P = .006) and occurred earlier (P = .022) in association with variants in genes other than MYBPC3. The inclusion of “genotype other than MYBPC3” and “presence of LGE” improved the predictive capacity of the models for the high-risk (C-statistic 0.94 vs 0.84 with HCM Risk-SCD; 0.88 vs 0.74 with ESC algorithm; 0.90 vs 0.80 with HCM Risk-Kids) and intermediate-risk categories (C-statistic 0.88 vs 0.51 with HCM Risk-SCD; 0.85 vs 0.64 with ESC algorithm; 0.84 vs 0.51 with HCM Risk-Kids).
ConclusionsThe predictive capacity of European risk models improves by incorporating the variables “genotype other than MYBPC3” and “presence of LGE”, although larger studies are required to validate their prognostic value.
Los modelos de estimación del riesgo de muerte súbita (MS) en miocardiopatía hipertrófica (MCH) pediátrica utilizados en nuestro medio no contemplan algunos parámetros de la práctica clínica habitual. El objetivo fue identificar factores de riesgo no clásicos y evaluar su valor pronóstico.
Pacientes y métodosEstudio observacional retrospectivo, incluyendo pacientes con MCH aislada de 0-18 años, evaluando variables clínicas, genéticas y de imagen. Se estimó el riesgo de MS o evento cardiaco arrítmico mayor (ECAM) según los tres modelos europeos más empleados, HCM Risk-SCD, algoritmo de la European Society of Cardiology (ESC) y HCM Risk-Kids, analizando su capacidad predictiva al añadir el genotipo y parámetros de imagen cardíaca avanzada.
ResultadosSe incluyeron 77 pacientes, seguimiento 5.25 años. 10 (13%) sufrieron un ECAM. La deformación miocárdica y el genotipo positivo mostraron asociación significativa con los ECAM, y tendencia la presencia de realce tardío (RT) en resonancia cardiaca (p = 0.062). Ocurrieron más eventos (HR = 18.5, p = 0.006) y más precoces (p = 0.022) con variantes en genes distintos a MYBPC3. La inclusión de “genotipo distinto a MYBPC3” y “presencia de RT” mejoró la capacidad predictiva de los modelos en las categorías de riesgo alto (estadístico-C 0.94 vs 0.84 en HCM-Risk-SCD; 0.88 vs 0.74 en algoritmo ESC; 0.90 vs 0.80 en HCM-Risk-Kids) e intermedio (estadístico-C 0.88 vs 0.51 HCM-Risk-SCD; 0.85 vs 0.64 guía ESC; 0.84 vs 0.51 HCM-Risk-Kids).
ConclusionesLa capacidad predictiva de los modelos de riesgo europeos mejora al incorporar las variables “genotipo distinto a MYBPC3” y “presencia de RT”, aunque se requieren estudios más amplios que permitan validar su potencial pronóstico.
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiomyopathy worldwide and the second most prevalent in children.1 A majority of cases result from pathogenic variants in genes encoding sarcomeric proteins (40%-60%), most frequently MYBPC3 and MYH72,3. Sudden cardiac death (SCD) is the leading cause of death in children and adolescents (annual incidence, 0.8%-2%) and is more frequent in cases of isolated HCM compared to cases occurring in the context of another disease/syndrome.4–6
The only effective preventive measure is the placement of an automated internal cardioverter-defibrillator (ICD), which is clearly indicated in patients that experience sustained ventricular tachycardia (VT) or have survived sudden cardiac arrest (SCA). In primary prevention, the risk factors for pediatric HCM have traditionally been extrapolated from the evidence in adults. However, in the past decade, risk prediction models have been developed to estimate the risk of SCD in isolated HCM that can be applied to children and adolescents.7–10 The HCM Risk-SCD model,11 the algorithm of the European Society of Cardiology (ESC) guideline for HCM12 and the HCM Risk-Kids model, specifically developed for the pediatric population,13 can be used to estimate the individualized risk of SCD within 5 years based on classic predictor variables such as age, maximal left-ventricular wall thickness (MLVWT) or severe left ventricular hypertrophy, left atrial diameter, left ventricular outflow tract (LVOT) gradient, family history of SCD, non-sustained ventricular tachycardia (NSVT) and unexplained syncope. Both the ESC guideline algorithm and the HCM Risk-Kids have been validated in children aged less than 16 years, while the HCM Risk-SCD was developed to assess individuals aged more than 16 years.10,14,15 The three models allow stratification of patients into three risk categories: low risk of SCD (ICD not indicated), intermediate risk (individualize management) or high risk (ICD recommended).11–13
In recent years, the use of advanced imaging techniques for assessment of HCM in pediatric patients has become widespread, and recent evidence suggests that some parameters, such as the presence of fibrosis detected by extensive late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) or features indicative of myocardial deformation (global longitudinal strain [GLS]) could offer added prognostic value.16,17 On the other hand, genetic testing of children with HCM is an increasingly common practice. A positive genotype allows confirmation of the disease, identification of HCM phenocopies that can benefit from specific treatment and assessment of family members.18,19 However, the prognostic implications of the identification of a pathogenic variant in pediatric HCM are not clear and genetic information is not included in the risk models currently used in our area.
With the working hypothesis that the precision of current prediction models can be optimized and that the estimated risk in the pediatric population depends on the applied model, we conducted a study to assess whether nonclassic predictor variables could increase the predictive value of existing risk prediction models, with particular emphasis on genotype data and the presence of myocardial fibrosis.
Sample and methodsWe conducted a retrospective longitudinal observational and inferential study in patients with isolated HCM (MLVWT z score > 2) diagnosed before age 18 years followed up in a reference center for the management of inherited heart diseases between February 2009 and August 2022.
We collected data on demographic, clinical, echocardiographic and genetic variables as well as cardiac stress testing parameters and the presence of LGE on CMR when the information was available. We also added the myocardial deformation value (GLS) if the technique had been performed.
Genetic testing was performed with panels that included at least 83 genes involved in primary and secondary HCM and, from 2019, clinical exome sequencing. The pathogenicity of the variants was classified according to the recommend dations of the American College of Medical Genetics.20 We defined positive genotype as the detection of at least one variant associated with the disease that was considered clinically relevant (pathogenic variants, likely pathogenic variants and variants of uncertain significance strongly suspected to be pathogenic).
We estimated the risk of SCD using the aforementioned models: the algorithm of the ESC guideline and the HCM Risk-Kids model in all patients, and the HCM Risk-SCD model in patients aged at least 16 years and/or weighing at least 55 kg.
We retrieved information on the cases in which an ICD was implanted for primary prevention and on the occurrence of SCD or a major arrhythmic cardiac event (MACE): SCA/SCD, appropriate ICD therapy or hemodynamically unstable sustained VT.
We analyzed the association of the incidence of SCD/MACE with classic risk factors and the additional variables: positive genotype, GLS and LGE (presence and extent expressed as the percentage of total left ventricular mass). We evaluated the predictive performance of the three models in the cohort before and after the addition of the nonclassic variables.
The statistical analysis was performed with the software R (version 4.2.3). To calculate the association between quantitative variables, we used the Wilcoxon rank test, and for qualitative variables, we used the Pearson χ2 test or the Fisher exact test. We analyze survival by means of Kaplan-Meier curve analysis. We used Cox regression to model risk over time as a function of the variables, using the hazard ratio (HR) to measure relative risk and the C-statistic at 5 years to measure goodness of fit. Statistical significance was defined as a p value of less than 0.05 for every test.
All patients or legal guardians signed an informed consent form to authorize the collection of clinical and genetic data, and the study was approved by the ethics committee of the hospital (CEIm 19/2019) and conducted in adherence to the principles of the Declaration of Helsinki.
ResultsBaseline characteristics of the cohort and incidence of sudden cardiac arrest or major arrhythmic eventWe identified 77 patients, with a median age at diagnosis of 9 years (3-12); most (90%) were asymptomatic at the time of the initial evaluation. Sixty-two percent (45/73) had a positive genotype. Forty-six percent had a variant in the MYBPC3 gene (n = 21) and 27% in the MYH7 gene (n = 12). The rest had variants in TPM1 (n = 5; 11%), TNNT2/TNNI3 (n = 3; 7%) or other genes (FHL1, ALPK3 and ACTC1; n = 4; 9%) (Appendix B, Table S1 of the Supplemental material). None of the patients carried a homozygous variant. One patient carried two relevant variants, one in MYH7 and one in MYBPC3, considered high-risk by the three calculators, the patient carried an ICD and had not experienced any MACEs during follow-up.
There were no statistically significant differences between patients with a positive genotype and patients with negative genetic testing results in any of the clinical or echocardiographic characteristics nor in the presence of myocardial fibrosis at diagnosis (Appendix B, Table S2 of the Supplemental material). During the follow-up, an ICD was implanted in 27 patients (35%), three for secondary prevention and 24 for primary prevention. All patients in the latter group had an intermediate to high risk according to the applied risk models.
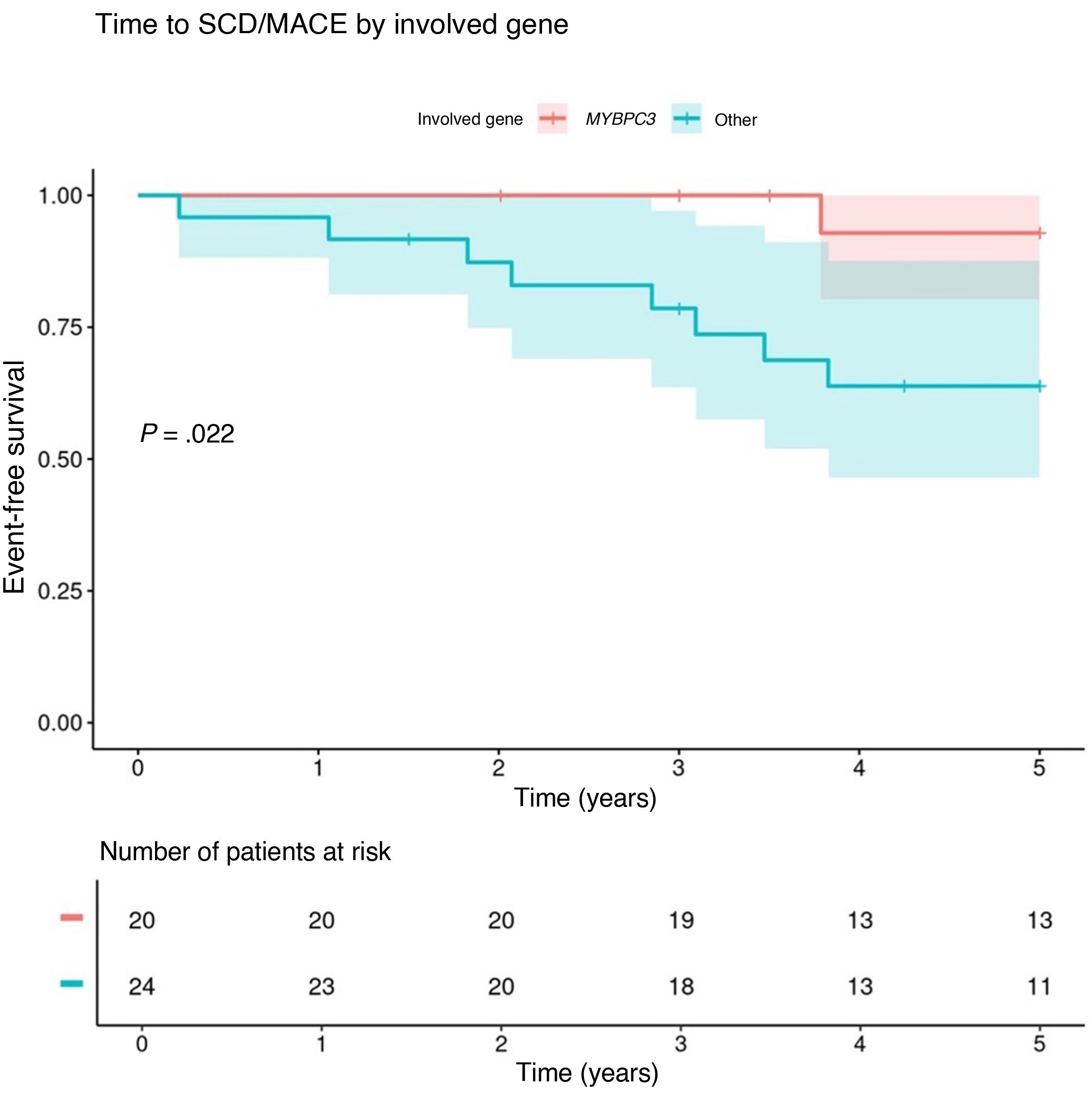
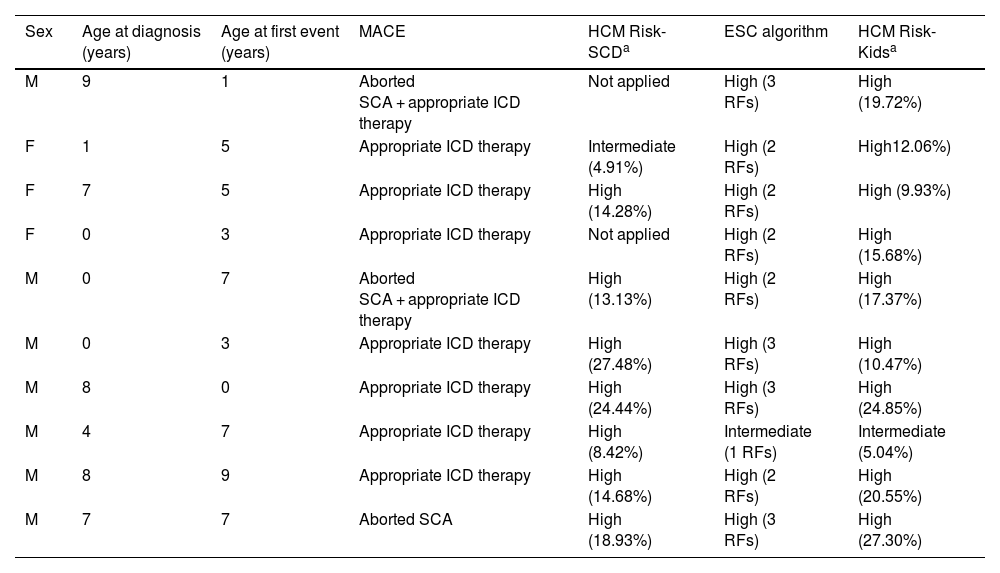
During the follow-up (median duration, 5.25 years [4.00-5.50]), 10 patients (13%) experienced at least 1 MACE (aborted SCA in one, appropriate ICD therapy in seven, and aborted SCA + appropriate ICD therapy in two). The rate of MACEs was of 2.6 cases per 100 patient-years (95% CI, 0.6-8.8), with a median age of 13.4 years (10.3-15.6) at the time of the first event. The median event-free survival from initiation of follow-up was 2.5 years. None of the patients died. Table 1 presents the 10 patients who experienced an event and the classification of the risk of arrhythmia at baseline according to the three risk models under study.
Description and assessment of baseline risk of arrhythmia in the 10 patients that experienced major arrhythmic events.
| Sex | Age at diagnosis (years) | Age at first event (years) | MACE | HCM Risk-SCDa | ESC algorithm | HCM Risk-Kidsa |
|---|---|---|---|---|---|---|
| M | 9 | 1 | Aborted SCA + appropriate ICD therapy | Not applied | High (3 RFs) | High (19.72%) |
| F | 1 | 5 | Appropriate ICD therapy | Intermediate (4.91%) | High (2 RFs) | High12.06%) |
| F | 7 | 5 | Appropriate ICD therapy | High (14.28%) | High (2 RFs) | High (9.93%) |
| F | 0 | 3 | Appropriate ICD therapy | Not applied | High (2 RFs) | High (15.68%) |
| M | 0 | 7 | Aborted SCA + appropriate ICD therapy | High (13.13%) | High (2 RFs) | High (17.37%) |
| M | 0 | 3 | Appropriate ICD therapy | High (27.48%) | High (3 RFs) | High (10.47%) |
| M | 8 | 0 | Appropriate ICD therapy | High (24.44%) | High (3 RFs) | High (24.85%) |
| M | 4 | 7 | Appropriate ICD therapy | High (8.42%) | Intermediate (1 RFs) | Intermediate (5.04%) |
| M | 8 | 9 | Appropriate ICD therapy | High (14.68%) | High (2 RFs) | High (20.55%) |
| M | 7 | 7 | Aborted SCA | High (18.93%) | High (3 RFs) | High (27.30%) |
Abbreviations: ESC, European Society of Cardiology; F, female; HCM, hypertrophic cardiomyopathy; ICD, implanted cardioverter defibrillator; Mmale; MAC, Emajor arrhythmic cardiac event; R, Frisk factor (no.); SCA, sudden cardiac arrest; SCD, sudden cardiac death.
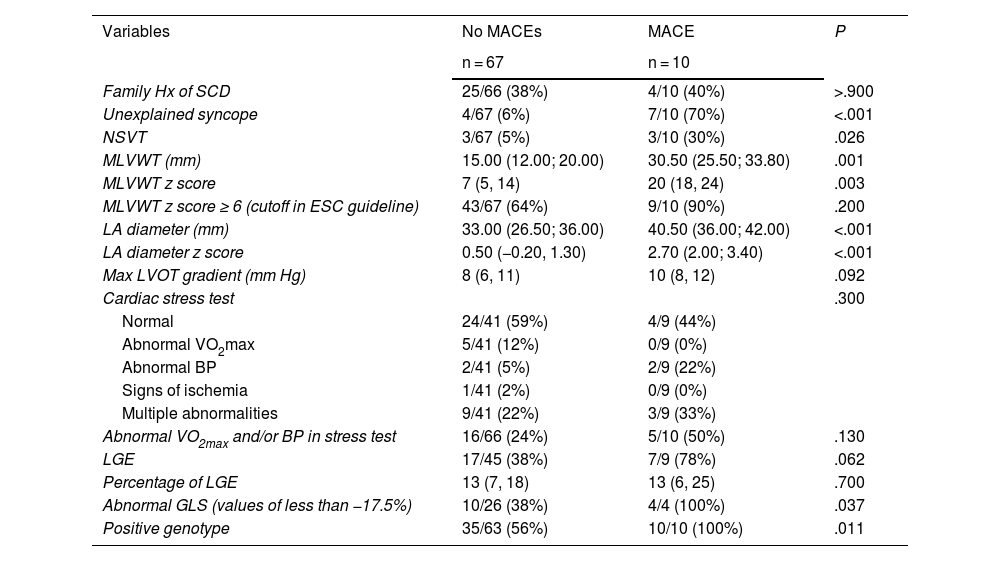
The analysis of the association of classic risk variables with the occurrence of MACEs identified significant associations with unexplained syncope, the NSVT gradient, the MLVWT z score and the left atrial diameter. We did not find an association with the maximal LVOT gradient nor the family history of SCD, which are considered risk factors in adults. On the other hand, the presence of an abnormal GLS and a positive genotype were significantly associated with the occurrence of MACEs. When it came to LGE, we found a greater prevalence of myocardial fibrosis in patients who experienced an event that did not reach statistical significance (Table 2).
Association of analyzed variables with the occurrence of a major arrhythmic cardiac events.
| Variables | No MACEs | MACE | P |
|---|---|---|---|
| n = 67 | n = 10 | ||
| Family Hx of SCD | 25/66 (38%) | 4/10 (40%) | >.900 |
| Unexplained syncope | 4/67 (6%) | 7/10 (70%) | <.001 |
| NSVT | 3/67 (5%) | 3/10 (30%) | .026 |
| MLVWT (mm) | 15.00 (12.00; 20.00) | 30.50 (25.50; 33.80) | .001 |
| MLVWT z score | 7 (5, 14) | 20 (18, 24) | .003 |
| MLVWT z score ≥ 6 (cutoff in ESC guideline) | 43/67 (64%) | 9/10 (90%) | .200 |
| LA diameter (mm) | 33.00 (26.50; 36.00) | 40.50 (36.00; 42.00) | <.001 |
| LA diameter z score | 0.50 (−0.20, 1.30) | 2.70 (2.00; 3.40) | <.001 |
| Max LVOT gradient (mm Hg) | 8 (6, 11) | 10 (8, 12) | .092 |
| Cardiac stress test | .300 | ||
| Normal | 24/41 (59%) | 4/9 (44%) | |
| Abnormal VO2max | 5/41 (12%) | 0/9 (0%) | |
| Abnormal BP | 2/41 (5%) | 2/9 (22%) | |
| Signs of ischemia | 1/41 (2%) | 0/9 (0%) | |
| Multiple abnormalities | 9/41 (22%) | 3/9 (33%) | |
| Abnormal VO2max and/or BP in stress test | 16/66 (24%) | 5/10 (50%) | .130 |
| LGE | 17/45 (38%) | 7/9 (78%) | .062 |
| Percentage of LGE | 13 (7, 18) | 13 (6, 25) | .700 |
| Abnormal GLS (values of less than −17.5%) | 10/26 (38%) | 4/4 (100%) | .037 |
| Positive genotype | 35/63 (56%) | 10/10 (100%) | .011 |
Abbreviations: BP, blood pressure; ESC, European Society of Cardiology; GLS, global longitudinal strain; Hx, history; LA, left atrium; LGE, late gadolinium enhancement; LVOT, left ventricular outflow tract; MACE, major arrhythmic cardiac event; MLVWT, maximal left ventricular wall thickness; NSVT, nonsustained ventricular tachycardia; SCD, sudden cardiac death; VO2max, maximum oxygen consumption.
In light of the association between having a positive genotype and the occurrence of MACEs, we conducted a Kaplan-Meier analysis of the development of MACEs based on the involved gene. Since MYBPC3 was the most frequently involved gene, we compared patients positive for MYBPC3 variants to patients with clinically significant variants in any other gene. We found a higher probability of events (HR = 18.5; 95% CI, 2.31-148; P = .006) and earlier occurrence of events in patients with clinically significant variants in genes other than MYBPC3 (Fig. 1).
Predictive ability of the risk prediction modelsAs can be seen in Table 1, none of the patients who experienced a MACE was classified as low risk at baseline with any of the risk prediction models. We found a significant association (P ≤ .005) between an increased risk score and the occurrence of MACEs in the three models under study, and the cumulative probability of experiencing an event increased with increasing predicted risk in the three models (Appendix B, Fig. S1 of Supplemental material).
Of the patients classified as high risk (in whom ICD is indicated), the percentage that experienced a MACE was 50% when the risk was assessed with the HCM Risk-SCD, 26% when it was assessed with the ESC algorithm and 33% when it was assessed with the HCM Risk-Kids.
Added predictive value offered by the addition of the genotype and advanced imaging parameters to the risk prediction modelsAfter the analysis of the risk prediction models in their current versions, we analyzed the genotype, the presence of LGA and abnormal GLS as possible variables to include in a pediatric HCM risk prediction model.
The univariate analysis found a significantly increased risk of experiencing a MACE over time if a patient was classified as high risk (P ≤ .02), with hazard ration values of 24.9 using the HCM Risk-SCD, 11.7 using the ESC algorithm and 18.8 using the HCM Risk-Kids.
On the other hand, in patients classified as having intermediate risk in the three models, compared to all other patients, the hazard ratio was less than 1, that is, these patients appeared to be less likely to experience a major event compared to the rest of the patients.
The risk of MACE associated with a positive genotype other than MYBPC3 in the cohort was 19 times higher compared to cases with variants in that gene (HR, 18.5; P = .006). On the other hand, the hazard ratio for experiencing a MACE attributable to the presence of LGE was 4.2 (P = .08).
Although we considered the GLS at the outset, it did not seem to be associated with the level of risk in the regression analysis, so we did not include it in subsequent analysis
We assessed the predictive value of the two nonclassic variables that we considered relevant enough for inclusion in the risk models under study by means of the C-statistic, with a value of 0.808 for the presence of a variant in a gene other than MYBPC3 and a value of 0.651 for the presence of LGE.
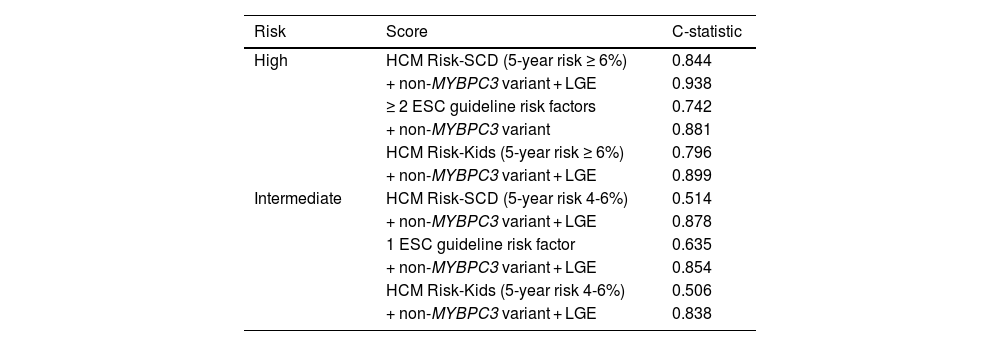
Table 3 presents the best (most accurate) configuration of each of the predictive models resulting from adding the selected nonclassic variables to the high- and intermediate-risk categories.
Multivariate analysis of the risk of SCD/MACE by in the cohor (Cox regression). Assessment of the predictive ability of the high and intermediate risk categories established with the three scores (HCM Risk-SCD, ESC Guidelines and HCM Risk-Kids) and adding the new variables (genotype and LGE).
| Risk | Score | C-statistic |
|---|---|---|
| High | HCM Risk-SCD (5-year risk ≥ 6%) | 0.844 |
| + non-MYBPC3 variant + LGE | 0.938 | |
| ≥ 2 ESC guideline risk factors | 0.742 | |
| + non-MYBPC3 variant | 0.881 | |
| HCM Risk-Kids (5-year risk ≥ 6%) | 0.796 | |
| + non-MYBPC3 variant + LGE | 0.899 | |
| Intermediate | HCM Risk-SCD (5-year risk 4-6%) | 0.514 |
| + non-MYBPC3 variant + LGE | 0.878 | |
| 1 ESC guideline risk factor | 0.635 | |
| + non-MYBPC3 variant + LGE | 0.854 | |
| HCM Risk-Kids (5-year risk 4-6%) | 0.506 | |
| + non-MYBPC3 variant + LGE | 0.838 |
Abbreviations: ESC, European Society of Cardiology; HCM, hypertrophic cardiomyopathy; LGE, late gadolinium enhancement; SCD, sudden cardiac death.
The behavior was similar for all 3 models. Although all patients classified in the highest risk groups would have an ICD implanted, the predictive ability of each model improved even more (C-statistic) by adding the presence of a clinically significant variant in a gene other than MYBPC3 and the presence of LGE in CMR. A finding that was of greater clinical interest was that the poor predictive ability of the independent variables that defined membership in the intermediate risk category improved clearly with the combined addition to the model of non-MYBPC3 variant and LGE variables.
DiscussionThe cumulative probability of SCD in the 5 years following diagnosis of isolated pediatric HCM is of 8%-10%.21 In our study, the frequency of MACEs was slightly greater (13%) over a follow-up period of 5.25 years. The rate of MACEs was of 2.6 cases per 100 patient-years, slightly greater than the one reported by the group that developed the HCM Risk-Kids model (1.5-2 cases per 100 patient-years).10,14 The corresponding SCD/MACE (at 5 years) was of 2.5 cases, compared to 1.5 in the HCM Risk-Kids study.13 In the group of patients who experienced events, the median time to the first event was 2.5 years, and the median age at first event was 13.4 years, similar to the findings of the PRIMaCY risk model study (median time to event of 2.2 years and median age at event of 14.5 years), which probably supports an increase in risk during adolescence.22,23
Although there is no clear evidence in the literature, genetic testing seems to offer a higher yield in pediatric compared to adult HCM.24 Our findings support this hypothesis: in our cohort, a disease-causing variant was identified in 6 out of 10 tested patients, which could guide prognostication and the family study.25
When it comes to myocardial deformation imaging techniques (strain analysis), while there was only evidence from a few cases, the findings suggest a potential prognostic value that may be worth validating. Few studies on the subject have been conducted to date, but some indicate a feasible correlation between conventional SCD markers and imaging parameters of left ventricular deformation.26
In addition, the frequency of LGE was double in the group of patients who experienced a MACE compared to those who did not, with a p value that neared the threshold of significance. Perhaps the association would be confirmed in a larger sample size, as suggested by studies of larger scope.27–29
The most recent ESC guidelines for the management of cardiomyopathies (2023) proposes that the presence of extensive LGE (≥15%) could be used to guide decision-making about prophylactic ICD implantation in patients classified as low or intermediate risk using the HCM Risk-SCD or HCM Risk-Kids scores.30 The Association for European Paediatric and Congenital Cardiology has suggested that, while the association between LGE and the risk of SCD has yet to be fully elucidated, along with the extent of LGE that would constitute a relevant risk, the presence of extensive LGE could be an additional risk factor for SCD in children and adolescents with HCM.9 In line with this, the updated guideline for the management of HCM of the American Heart Association, published in 2024, included a section on SCD risk assessment in children and adolescents proposing that consideration of additional factors such as LGE or signs of systolic dysfunction may be useful in the management of these patients.31
We found a significant association between a positive result of genetic testing and the occurrence of MACEs in the cohort (P = .011). In fact, all the events occurred in patients with a positive genotype. These findings support the previous findings of the PRIMaCY study, which established a prediction model that, as a novelty, included the presence of pathogenic variants.22
The higher frequency and earlier occurrence of events observed in our study in patients with variants of genes other than MYBPC3 were consistent with previous reports in the literature, with evidence of an association between the presence of pathogenic variants in MYBPC3 with milder phenotypes and later onset.25 These findings support consideration of a greater importance of the genotype in risk stratification.25,32,33 Additional studies in larger samples are required to make a similar assessment of the prognostic value of MYH7 variants and double variants.
In the cohort, the analyzed predictive models seemed effective at predicting events, although their results did not exactly agree in every case and, furthermore, the majority of patients classified as high risk did not suffer major events during the follow-up. Thus, it seems reasonable to improve and refine predictive algorithms, and the inclusion of new variables could be valuable to this end.
Similarly to a study of risk in pediatric HCM conducted in Sweden, which compared the performance of the HCM Risk-Kids score alone versus the HCM Risk-Kids combined with other variables (specifically the electrocardiogram risk score),34 we carried out multivariate analyses to estimate the predictive ability of the different models under study with the addition of the proposed nonclassic variables.
The predicted risk was lower in the intermediate risk group compared to all other patients using any of the three scores (which makes clinical sense, as it was compared with all other patients, including those with higher risk), with a C-statistic that reflected a poor discriminative ability. The predictive ability improved significantly when a patient was classified as intermediate risk, carried a variant in a gene other than MYBPC3 and exhibited LGE on CMR. We ought to highlight the significant association and predictive ability of the presence of variants in genes other than MYBPC3.25
The main limitations of the study are its single-center design and the limited number of events it included (as expected given the low frequency of the disease, and despite the inclusion of cases from the entire national territory). In regard to the statistical analysis (lack of fit in the model), it we were unable to include the combined intermediate-high risk group, or compare the intermediate risk to the low risk group or the low risk group to the rest of groups in the regression analysis, so we could not analyze the predictive value that the new variables would add in the assessment of these groups.
ConclusionIn the studied cohort of pediatric patients with HCM, a positive phenotype and an abnormal GLS were significantly associated with the occurrence of MACEs. The analysis of the presence of LGE showed a similar tendency that did not reach statistical significance. In addition, MACEs were more frequent and occurred earlier in patients with disease-causing variants in genes other than MYBPC3.
In patients classified as high or intermediate risk using the SCD HCM Risk-SCD, ESC guideline or HCM Risk-Kids, the predictive ability of the risk prediction model improved with the combined addition of the “positive genotype other than MYBPC3” and “presence of late gadolinium enhancement” variables.
These results demonstrate the relevance of these variables for prediction of arrhythmic risk and evince the need to perform multicenter studies to enable the development of new SCD risk calculators for pediatric HCM.
FundingThis research project did not receive specific financial support from funding agencies in the public, private or not-for-profit sectors.
The following are Supplementary data to this article:
Predictive ability of the applied risk prediction models. (A) Association of results obtained with the HCM Risk-SCD, ESC algorithm and HCM Risk-Kids with the occurrence of SCD/MACE during follow-up. (B) (C) (D) Cumulative probability of a MACE at 5 years by risk group based on (B) HCM Risk-SCD, (C) ESC algorithm, (D) HCM Risk-Kids.