The incidence of paediatric inflammatory bowel disease has increased in recent decades. The aim of the present study was to evaluate the role of proactive and serial monitoring of tumour necrosis factor (TNF) inhibitor levels to maintain clinical remission and mucosal healing in the follow-up of paediatric patients with Crohn disease (CD).
MethodsProspective study that included all patients diagnosed with CD and treated with adalimumab or infliximab between May 2015 and November 2020 who underwent serial and proactive monitoring of TNF inhibitor levels.
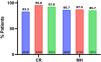
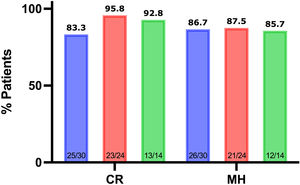
ResultsThe study included 30 patients, 21 male (70%). The mean age at diagnosis was 11.3 years (SD, 2.0), the mean age at initiation of TNF inhibitors was 12.6 years (SD, 1.9) with a mean duration of follow-up of 27.1 ± 9.1 months. Clinical remission was defined as a weighted Pediatric Crohn’s Disease Activity Index (wPCDAI) of less than 12.5 and mucosal healing as a Mucosal Inflammation Non-invasive Index (MINI) of less than 8. During the follow-up, patients were in clinical remission in 87.1% of the visits, presented with mild disease in 11.4% and with moderate disease in 1.5%, and mucosal healing was assumed in 83% of the visits. The rates of clinical remission and mucosal healing at 1, 2, and 3 years of follow-up were 83.3%, 95.8%, 92.8%, and 86.7%, 87.5% and 85.7%, respectively.
ConclusionsProactive and serial monitoring of serum TNF inhibitor levels may make it possible for patients to maintain clinical remission and mucosal healing in the maintenance phase, with individualised optimization of the required dosage and minimization of secondary loss of response.
La incidencia de la enfermedad inflamatoria intestinal pediátrica ha aumentado en las últimas décadas. El objetivo del presente estudio fue evaluar el papel de la monitorización proactiva y en serie de los niveles de fármacos anti-TNF (factor de necrosis tumoral) para mantener la remisión clínica y la curación mucosa durante el seguimiento de pacientes pediátricos con Enfermedad de Crohn (EC).
MétodoEstudio prospectivo que incluye a todos los pacientes diagnosticados de EC y tratados con adalimumab o infliximab entre mayo de 2015 y noviembre de 2020, en los que se ha realizado una monitorización seriada y proactiva de los niveles de anti-TNF.
ResultadosSe incluyeron treinta pacientes, 21 varones (70%). La edad en el momento del diagnóstico fue de 11,3 ± 2,0, la edad en el momento de iniciar el anti-TNF fue de 12,6 ± 1,9 años con un tiempo medio de seguimiento de 27,1 ± 9,1 meses. Se consideró remisión clínica si wPCDAI < 12,5 puntos y curación mucosa si el índice MINI < 8. Durante el seguimiento, el paciente estuvo en remisión clínica en el 87,1% de las visitas, el 11,4% presentó enfermedad leve, el 1,5% enfermedad moderada y se asumió curación mucosa en el 83% de las visitas. Las tasas de remisión clínica y mucosa tras 1, 2 y 3 años de seguimiento fueron 83,3%, 95,8%, 92,8% y 86,7%, 87,5% y 85,7%, respectivamente.
ConclusionesLa monitorización proactiva y seriada de los niveles séricos de anti-TNF podría permitir al paciente mantener la remisión clínica y la curación mucosa durante el seguimiento, optimizando individualmente la dosis requerida y minimizando la pérdida secundaria de respuesta.
Crohn disease (CD) is a chronic inflammatory disease characterized by segmental and transmural involvement of any part of the gastrointestinal tract. It is a form of inflammatory bowel disease (IBD), an umbrella term which also comprises ulcerative colitis (UC) and inflammatory bowel disease unclassified (IBDU). The incidence of paediatric IBD has increased in recent decades, both in Spain1,2 and worldwide.3 In recent years, different strategies have been implemented, structured into 3 main domains: diagnosis, follow-up goals and therapeutic management, with the ultimate goal of modifying the course of the disease.4
The development of tumour necrosis factor (TNF) inhibitors has brought a significant advance in the treatment of these patients, modifying the prognosis in the short, medium and long terms,5 especially when these drugs are prescribed in the optimal time window. In paediatric patients, the identification of factors associated with a poor prognosis may indicate the need of early initiation of anti-TNF therapy to improve outcomes.6 The latest guideline of the European Crohn’s and Colitis Organisation (ECCO) and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) mention this aspect.7 The goals of treatment go beyond corticosteroid-free clinical remission. Mucosal healing (MH) and transmural healing (TH), deep remission (MH + TH), prevention of bowel damage and the resulting disability and the improvement of health-related quality of life are particularly important objectives, as is clinical remission. Achieving these goals requires close monitoring of patients with frequent clinical evaluations.4
Therapeutic drug monitoring (TDM) involves periodic measurement of serum drug and anti-drug antibody concentrations. It can be reactive, if it is practiced in response to events that have already occurred (clinical relapse, infusion reaction, development of side effects, etc), proactive, if its performed with the aim of achieving pre-established targets, or predictive, if it is performed with the aim of anticipating events. Using the doses indicated in the pivotal trials (REACH8 and IMAgINE19) would yield similar remission outcomes, so new strategies should be explored to improve outcomes. There is some controversy regarding the efficacy, efficiency10,11 and effectiveness of proactive monitoring of serum levels of TNF inhibitors in the many scenarios that can occur during treatment with these agents.12 During induction therapy, serum levels of TNF inhibitors can be used to determine the risk of secondary loss of response in early stages due to the development of antibodies against the TNF inhibitor.13 Anti-drug antibody (ADA) formation and delays in therapeutic decision-making can have an impact in subsequent disease outcomes. In this sense, proactive monitoring of anti-TNF drug concentrations would allow early detection of ADA formation and implementation of strategies aimed at avoiding secondary loss of response.12,14
The use of proactive TDM reduces the need for combination therapy,14 restricting it to the management of more serious, transient situations with increased drug clearance. At present, when ELISA-type kits cannot simultaneously quantify drug and antibody levels, proactive monitoring of TNF inhibitor serum levels may show such a progressive decrease when neither the dose nor the interval between doses have changed, which, in the absence of enhanced drug clearance, could reflect the development of ADAs.15
Clinical activity indices are only moderately correlated to the degree of intestinal mucosal inflammation, so they are not adequate tools to be used in isolation to make decisions regarding the management of the patient.16 In this context, the Mucosal Inflammation Non-invasive Index (MINI) is a tool that allows the evaluation of mucosal inflammation in children with CD, identifying those with MH with a high sensitivity (84%) and specificity (87%),17 and therefore may be a useful indicator for the purpose of TDM. We present the long-term results of proactive monitoring of TNF inhibitor concentrations in patients with CD.
MethodsWe conducted a prospective observational descriptive study between May 2015 and November 2020. We included all patients with a CD diagnosis treated with TNF inhibitors, performing serial and proactive measurement of serum concentrations of adalimumab (ADL) (Humira, AbbVie Deutschland GmbH& Co. KG) and infliximab (IFX) (Remicade, Janssen Biologics BV) during the maintenance phase. The diagnosis of CD was established applying the Porto criteria,18 and the phenotype at diagnosis based on the Paris classification.19 We excluded patients with UC, with IBDU or with severe perianal disease requiring surgical treatment.
The weight (kg) and height (cm) of the patient were measured at each visit with the patient barefoot and in underwear. We calculated weight, height, and body mass index (BMI) z-scores were calculated using Spanish growth charts as reference (https://www.seghnp.org/nutricional/).20
We also calculated the weighted Pediatric Crohn’s disease activity index (wPCDAI)16 and the MINI.17 We defined clinical remission as a wPCDAI of less than 12.5, and mucosal healing as a MINI of less than 8. We studied maintenance doses used in the pivotal studies (REACH and IMaGINe1): 5 mg/kg every 8 weeks for IFX8 and 40 mg every 2 weeks for ADL.9 Secondary loss of response, also known as secondary non-response, refers to patients who respond after induction but subsequently stop responding during maintenance therapy.21 The mechanisms underlying this loss of response can be pharmacokinetic (subtherapeutic drug concentrations in the absence of ADAs), pharmacodynamic (adequate drug concentrations) or immunogenic (subtherapeutic drug concentrations in the presence of ADAs).22
We defined clinical remission (wPCDAI < 12.5) in the context of C-reactive protein (CRP) concentrations of 5 mg/L or greater as silent CD.23,24 Blood and faecal samples were collected on the same day before drug administration. The evaluation also included a complete blood count (CBC), and determination of CRP, albumin, faecal calprotectin, erythrocyte sedimentation rate (ESR) and serum concentrations of IFX and ADL (proactive monitoring of drug levels).
The measurement of IFX, ADL and ADAs concentrations was carried out using an ELISA test (Promonitor, Grifols). The assay ranges for the Promonitor-IFX and the Promonitor-ADL tests were 0.035 µg/mL to 14.4 µg/mL and 0.024 µg/mL to 12 µg/mL, respectively. The assay ranges for the Promonitor-Anti-IFX and the Promonitor-Anti-ADL tests were 5–1440 AU/mL and 10–2000 AU/mL, respectively. We measured faecal calprotectin with Calprest (Eurospital), an ELISA kit that uses polyclonal antibodies with a measurement range of 15.6–500 µg/g.
The treatment regimen was prescribed based on the judgment of the physician in charge of the patient, without pre-specified TNF inhibitor target concentrations for dose adjustments, although other parameters were taken into account in decision making (clinical features, blood test results or faecal calprotectin values). In the maintenance phase, serum levels of IFX were measured each time the patient came in to undergo drug infusion, and serum levels of ADL were measured 12 weeks apart or earlier if there was any reason to suspect a relapse.
We assessed adherence to treatment based on the dispensing records of the pharmacy department of the outpatient clinic by calculating the medication possession ratio (MPR): (number of dosage units dispensed – number of dosage units returned)/number of dosage units prescribed × 100.
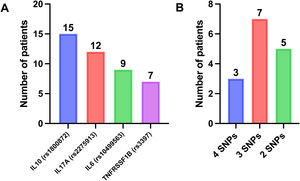
Single-nucleotide polymorphisms (SNPs)As done in previous studies,25–28 we evaluated the presence of SNPs associated with long-term response to IFX and ADL in CD in 15 patients. The TNFRSF1B C allele (rs3397); the IL10 CC genotype (rs1800872); the IL17A G allele (rs2275913) and the C allele in IL6 (rs10499563) have been identified as predictors of a longer-term response to TNF inhibitors.27
Statistical analysisWe have expressed variables as mean and standard deviation (SD) if the data followed a normal distribution, and otherwise as median and interquartile range (IQR). We used the Kolmogorov-Smirnov test to assess normality of the distribution. We used the Student t-test and Wilcoxon signed-rank test to compare paired samples and the χ2 test to compare proportions. The correlation between variables was assessed by means of the Spearman correlation coefficient. We performed a receiver operating characteristics (ROC) curve analysis to calculate the TNF inhibitor concentration cut-off points for clinical remission and mucosal healing. We considered P values of less than .05 statistically significant. The statistical analysis was conducted with the software SPSS version 25, and graphs generated with Prism 9 for macOS, version 9.2.0 (283), July 15, 2021.
ResultsThe study included 30 patients, 21 of them male (70%), of who 24 received treatment with ADL and 6 with IFX. The age at diagnosis was 11.3 years (SD, 2.0) and the age at the time anti-TNF therapy initiation 12.6 years (SD, 1.9). Table 1 presents the clinical and demographic characteristics of the patients.
Baseline characteristics of patients with Crohn disease treated with anti-TNF therapy (N = 30).
| Total (N = 30) | ADA (n = 24) | IFX (n = 6) | P | |
|---|---|---|---|---|
| Male sex, n (%) | 21 (70) | 17 (70.8) | 4 (66.6) | .028 |
| Age at diagnosis (years) | 11.3 ± 2.0 | 11.1 ± 2.1 | 11.8 ± 1.3 | .561 |
| Time from onset of symptoms to diagnosis (m) | 4 (IQR, 2.2−7) | 4.1 (IQR, 2.2−6.9) | 3.3 (IQR, 2.3−4.9) | .477 |
| Age at anti-TNF initiation | 12.6 ± 1.9 | 12.7 ± 1.9 | 12.4 ± 1.8 | .743 |
| Time from diagnosis to anti-TNF (m) | 9.9 (IQR, 2.8−21.5) | 10.3 (IQR, 4.4−24.1) | 3.7 (IQR, 0.05−14.6) | .143 |
| Paris classification19at diagnosis | n (%) | n (%) | n (%) | |
| A1b | 25 (83.3) | 20 (83.3) | 5 (83.3) | |
| A1a | 5 (16.7) | 4 (16.7) | 1 (16.7) | .731 |
| L3L4a | 13 (43.3) | 9 (37.5) | 4 (66.7) | .099 |
| L3L4b | 1 (3.3) | – | 1 (16.7) | |
| L1 | 8 (26.7) | 7 (29.1) | 1 (16.7) | |
| L3 | 4 (13.3) | 4 (16.7) | – | |
| L2 | 2 (6.6) | 2 (8.3) | – | |
| L1L4b | 1 (3.3) | 1 (4.1) | – | |
| L1L4a | 1 (3.3) | 1 (4.1) | – | |
| B1 | 27 (90) | 22 (91.6) | 5 (83.3) | .515 |
| B2 | 3 (10) | 2 (8.4) | 1 (16.7) | |
| Perianal disease (p) | 11 (36.6) | 8 (33.3) | 3 (50) | .408 |
| Growth retardation (G1) | 5 (16.6) | 3 (12.5) | 2 (33.3) | .269 |
| Treatment before anti-TNF | n (%) | n (%) | n (%) | |
| EEN | 28 (93.3) | 23 (95.8) | 5 (83.3) | .366 |
| AZA | 28 (93.3) | 22 (91.6) | 6 (100) | .634 |
| MTX | 4 (13.3) | 4 (16.6) | – | .499 |
| 6MP | 2 (6.7) | 1 (4.1) | 1 (16.6) | .366 |
| Steroids | 12 (40) | 11 (45.8) | 1 (16.6) | .237 |
| Anthropometry at start of anti-TNF | ||||
| Weight (kg) | 39.7 (IQR, 33.6−47.6) | 41.2 (IQR, 36.2−48.1) | 34.1 (IQR, 28.3−40.2) | .078 |
| Weight z-scoreA | −0.75 (IQR, −1.4 to 0.08) | −0.42 (IQR, −1.3 to 0.13) | −1.1 (IQR, −1.9 to −0.02) | .259 |
| Height (cm) | 148 (IQR, 140.5−160.4) | 152.5(IQR, 141.7−163.7) | 141 (IQR, 134.8−155.8) | .140 |
| Height z-scoreA | −0.39 (IQR, −1.16 to 0.76) | −0.2 (IQR, −1.06 to 0.78) | −0.52 (IQR, −2.5 to −0.34) | .125 |
| BMI (kg/m2) | 17.8 (IQR, 15.5−20.4) | 18.3 (IQR, 15.8−20.5) | 16.1 (IQR, 14.1−19.4) | .316 |
| BMI z-scoreA | −0.7 (−1.1 to 0.53) | −0.38 (IQR, −1.1 to 0.54) | −1.0 (−1.5 to −0.40) | .316 |
| Baseline wPCDAIB | n (%) | n (%) | n (%) | .086 |
| Remission | 8 (9.1) | 7 (29.1) | 1 (16.7) | |
| Mild | 10 (18.2) | 9 (37.5) | 1 (16.7) | |
| Moderate | 7 (31.8) | 5 (20.8) | 2 (33.3) | |
| Severe | 5 (40.9) | 3 (12.5) | 2 (33.3) | |
| Concomitant treatment with anti-TNF | n (%) | n (%) | n (%) | |
| AZA | 20 (66.7) | 17 (70.8) | 3 (50) | .306 |
| PEN | 19 (63.3) | 16 (66.7) | 3 (75) | .380 |
| Steroids | 4 (13.3) | 3 (12.5) | 1 (16.7) | .612 |
| MTX | 1 (3.3) | 1 (4.1) | – | .793 |
| 6MP | 4 (13.3) | 1 (4.1) | 3 (50) | .169 |
| 5-ASA (oral) | 1 (3.3) | 1 (4.1) | – | .8 |
| Baseline MINI | 13 (IQR, 9.5−14) | 11 (7−13) | 16 (10.5−17) | .243 |
| Baseline laboratory results | Total (N = 30) | ADA (n = 24) | IFX (n = 6) | |
| Faecal calprotectin (μg/g) | 623 (IQR, 419−1410) | 623 (IQR, 408−1290) | 752 (IQR, 429−4206) | .437 |
| CRP (mg/L) | 20.3 (IQR, 13−30.6) | 16.2 (IQR, 12−28.5) | 38.4 (IQR, 15.9−66.9) | .102 |
| ESR (mm/h) | 35 (IQR, 17−45) | 35 (IQR, 14−38) | 42 (IQR, 20.2−86.2) | .121 |
| Albumin (g/dL) | 3.6 (IQR, 3.2−4.2) | 3.5 (IQR, 3.2−4.3) | 3.7 (IQR, 2.0−3.9) | .345 |
| Vitamin D (ng/mL) | 21.8 (IQR, 18.0−28.9) | 18 (IQR, 10−25) | 22 (IQR, 9−27) | .865 |
| Hb (g/dL) | 11.9 (IQR, 10.9−12.8) | 11.9 (IQR, 10.3−12.8) | 11.3 (IQR, 11.1−12.3) | .667 |
| Htc (%) | 36.6 (IQR, 35.1−38.5) | 36.8 (IQR, 34−38.7) | 36.2 (IQR, 35.5−37.7) | .820 |
| WBC (×109/L) | 7.4 ± 2.2 | 7.4 ± 2.4 | 7.4 ± 1.7 | .743 |
| Platelets (×109/L) | 424 ± 116 | 413 ± 116 | 469 ± 117 | .321 |
AZA, azathioprine; CRP, C-reactive protein; EEN, exclusive enteral nutrition; ESR, Erythrocyte sedimentation rate; Hb, haemoglobin; Hct, haematocrit; IQR, Interquartile range; MINI, Mucosal Inflammation Non-Invasive Index17; MTX, methotrexate; PEN, partial enteral nutrition; WBC, white blood cell count; wPCDAI, weighted Paediatric Crohn’s Disease Activity Index; 5-ASA, 5-aminosalicylic acid; 6MP, 6 mercaptopurine.
Although all 30 patients were offered initial treatment with ADL, 6 refused it on account of a fear of needles. The 6 patients treated with IFX received an induction dose of 5 mg/kg on days 0, 2 and 6 of treatment and every 8 weeks thereafter. In the ADL group, 2 patients received an induction dose of 80/40 mg because their weight was under 30 kg, and the rest (22 patients) an induction dose of 160/80 mg. The median ADL dose per kilogram of body weight was 3.9 mg (IQR, 3.3–4.3) for the first dose and 1.9 mg (IQR, 1.6–2.1) for the second. The median dose per square meter was 118 mg (IQR, 103–130) for the first dose and 59.1 mg (IQR, 51–65) for the second. The mean duration of follow-up was 27.1 months (SD, 9.1) (28.9 ± 8.4 months for IFX vs 26.6 ± 9.3 for ADL; P = .556). During the study period, patients made a total of 218 visits, 77 in the group treated with IFX (12.8 visits per patient) and 141 in the group treated with ADL (5.8 visits per patient). There were no cases of loss of response or discontinuation of treatment due to poor adherence.
TNF inhibitor dosage during the follow-upIn the overall sample, 2 of the 6 patients treated with IFX and 13 of the 24 treated with ADL (33.3% vs 54.1%; P = .651) maintained the dose of 5 mg/kg/8 weeks for IFX or the 40 mg/2 weeks for ADL throughout the follow-up. In the ADL group, there were no significant differences in the duration of follow-up between who required dose adjustments and those who did not (24.5 ± 8.5 months vs 30.9 ± 9.3 months; P = .091). In 48.6% of the visits made during the study period, the dose and dose intervals of the treatment prescribed to patients were consistent with those used in the pivotal studies. Stratifying by TNF inhibitor, this was only the case in 15.6% of patients treated with IFX, compared to 66.7% of those treated with ADL (P < .0001). Table 2 presents the treatment regimens.
Infliximab and adalimumab doses and intervals and their frequency in episodes.*
| Scheme | n (%) | |
|---|---|---|
| Infliximab | 5 mg/kg/8 wk | 12 (15.6%) |
| 7.5 mg/kg/6 wk | 12 (15.6%) | |
| 10 mg/kg/4 wk | 12 (15.6%) | |
| 7.5 mg/kg/4 wk | 11 (14.3%) | |
| 12 mg/kg/4 wk | 9 (11.7%) | |
| 10 mg/kg/6 wk | 9 (11.7%) | |
| 7.5 mg/kg/8 wk | 5 (6.5%) | |
| Other | 7 (9%) | |
| Total | 77 (100%) | |
| Adalimumab | 40 mg/2 wk | 94 (66.7%) |
| 40 mg/wk | 34 (24.1%) | |
| 80 mg/wk | 7 (4.9%) | |
| 20 mg/2 wk | 5 (3.6%) | |
| Other | 1 (0.7%) | |
| Total | 141 (100%) |
wk, week.
We evaluated the effect of concomitant therapy with thiopurines (azathioprine/6-mercaptopurine) or methotrexate on the need to adjust the TNF inhibitor dose or dose interval. Overall, the dose was adjusted to the dose used in the pivotal studies in 45.5% of the visits corresponding to patients receiving combination therapy compared to 53.5% of visits of patients treated with monotherapy (P = .138). In the group treated with ADL, concomitant treatment with an immunomodulator (IMM) (azathioprine/6-mercaptopurine or methotrexate) was associated with a greater number of visits with the 40 mg/2 weeks dose (85.1% vs 61.2%; P = .001), a difference that was not observed in the group treated with IFX (12.5% vs 2.2%; P = .092).
Laboratory turnaround timesThe turnaround time was 7 days (IQR, 6–14 days) for IFX levels, 9 days (IQR, 4–18) for ADL levels, and 4 days (IQR, 2–8 days) for faecal calprotectin. The results of all other laboratory tests were available within 48 h.
Clinical remission, silent disease and secondary loss of responseDuring the follow-up, patients were in clinical remission in 87.1% of the visits, presented mild disease in 11.4% and presented moderate disease in 1.5%. In 22 of the 190 visits in which the patient was in clinical remission (11.5%) the CRP concentration was 5 mg/L or greater (silent CD). Secondary loss of response due to a pharmacokinetic mechanism was identified in 7.3% of the visits, with no differences based on the TNF inhibitor (6.5% in IFX group vs 7.6% in ADL group; P = .513), and recovery of response was achieved by increasing the dose or shortening the interval between doses. During the follow-up, there were no cases of secondary loss of response due to immunogenic or pharmacodynamic causes or of cessation of treatment. None of the patients required addition of steroid therapy to achieve clinical remission.
Two patients presented a progressive decrease in TNF inhibitor concentrations in the absence of increases in CRP or faecal calprotectin levels, which the physician in charge, after verifying the correct administration of the treatment, could attribute to the development of ADAs not quantifiable by the detection test used. In both cases, after treatment intensification, TNF inhibitor levels normalized and remained within range without need of further optimization.
At 12 months of anti-TNF therapy, 83.3% of the patients were in steroid-free remission, at 2 years, 95.8%, and at 3 years, 92.8% (Fig. 1).
In addition, an improvement in faecal calprotectin, albumin, CRP, ESR, haemoglobin, and vitamin D values was observed during follow-up in relation to baseline values at the start of anti-TNF therapy (Table 3).
Outcomes to up to 3 years of follow-up.
| Baseline, n = 30 | 1 year, n = 30 | 2 years, n = 24 | 3 years, n = 14 | |
|---|---|---|---|---|
| Faecal calprotectin (μg/g) | 623 (419−1410) | 142 (32−254) | 131 (20−389) | 158 (37−458) |
| CRP (mg/L) | 20.3 (13−30.6) | 2.9 (2.9−5.6) | 2.9 (2.9−2.9) | 2.9 (2.9−16.8) |
| ESR (mm/h) | 35 (17−45) | 12 (4−16) | 10 (4−16) | 10 (3.5−22) |
| Albumin (g/dL) | 3.6 (3.2−4.2) | 4.3 (3.5−4.5) | 4.2 (3.9−4.4) | 4.1 (3.7−4.3) |
| Vitamin D (ng/mL) | 21.8 (18.0−28.9) | 25.9 (23.1−32.4) | 28.6 (21.8−31.6) | 28.3 (17.4−34.1) |
| Hb (g/dL) | 11.9 (10.9–12.8) | 13.3 (12.2−13.9) | 13.8 (12.7−14.8) | 31.3 (12.4−14.7) |
| Htc (%) | 36.6 (35.1−38.5) | 39.5 (36.2−42.6) | 42.2 (39.4−43.7) | 40.1 (39.2−43.6) |
| WBC (x109/L) | 7.4 ± 2.2 | 6.4 ± 1.6 | 6.9 ± 1.5 | 6.5 ± 0.8 |
| Platelets (x109/L) | 424 ± 116 | 263 ± 82 | 261 ± 72 | 270 ± 54 |
| Clinical remission | 25 (83.3%) | 23 (95.8%) | 13 (92.8%) | |
| Mucosal healing | 26 (86.7%) | 21(87.5%) | 12 (85.7%) |
Remission: wPCDAI < 12.516; mucosal healing: MINI < 8. Data are presented as median (interquartile range), mean ± standard deviation or n (%).
CRP, C-reactive protein; Hb, haemoglobin; Hct, haematocrit; ESR, erythrocyte sedimentation rate; IQR, interquartile range; WBC, white blood cell count.
In the overall sample, the median MINI score obtained during the follow-up was 4 (IQR, −3 to 7), with MH being identified in 83% of visits. The results are shown in Fig. 1. The correlation between the MINI and wPCDAI scores was low: 0.298 (P < .0001).
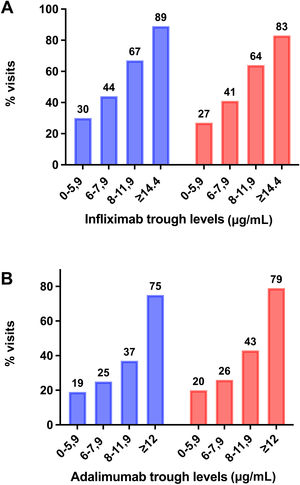
Drug levels for clinical remission and mucosal healingFig. 2 shows the percentage of visits in which the patients were in clinical remission or exhibited MH in relation to TNF inhibitor levels.
For IFX, a serum level of 5.85 µg/mL predicted clinical remission (area under the curve [AUC], 0.614 [0.405−0.823]; P = .328) with a sensitivity of 68.4% and a specificity of 57.1%, and levels of 7.30 µg/mL predicted MH (AUC, 0.584 [0.377−0.791]; P = .383), with a sensitivity of 58.5% and a specificity of 54.5%.
For ADL, a serum level of 9.4 µg/mL predicted clinical remission (AUC, 0.626 [0.478−0.774]; P = .082) with a sensitivity of 61.7% and a specificity of 63.2%, and levels of 9.7 µg/mL predicted MH (AUC, 0.464 [0.347−0.580]; P = .562) with a sensitivity of 59% and a specificity of 37%.
Adherence to treatmentWe found an overall mean adherence of 90.5% (SD, 6.8). In the ADL group, the mean adherence was 92.2% (SD, 5.9) and in the IFX group, 85.7% (SD, 8.1) (P = .527).
SNPs associated with a long-term response to TNF inhibitorsIn all 15 patients who underwent a pharmacogenetic study, testing detected at least 2 SNPs associated with a long-term response to TNF inhibitors (Fig. 3).
DiscussionOur results show that proactive monitoring of TNF inhibitor levels combined with the other inflammatory parameters is an efficient practice, as it reduces the risk of clinical and laboratory relapse and of secondary loss of response due to immunogenic or pharmacokinetic mechanisms, in addition to allowing early identification of silent CD in paediatric patients. The updated ECCO-ESPGHAN guideline recommends its implementation, establishing target trough levels during the maintenance phase to achieve MH of 5 µg/mL for IFX and 7.5 µg/mL for ADL.7 In our study, there were no predetermined target TNF inhibitor trough levels.
The Paediatric Crohn’s Disease Adalimumab Level-Based Optimization Treatment (PAILOT) trial compared the proactive approach to reactive treatment with ADL in the paediatric population, and found that steroid-free remission was more frequent in the proactive group (82% vs 48%; P = .002). The authors established a target ADL trough level of 5 μg/mL.14 Although this approach may be appropriate, it is not the usual approach in real-world practice, in which clinical and analytical data have to be considered in addition to drug concentrations, as we did in our study.
Similar to the results of the study published by Ungar et al.,29 in our sample we found that a subset of patients in clinical remission and with MH had concentrations of less than 5 µg/mL, in support of our approach to proactive monitoring, which is not based on predetermined target levels. In another retrospective series of paediatric patients, Lyles et al. reported that proactive monitoring was more effective in achieving steroid-free remission at 52 weeks of treatment compared to a reactive approach. They found that proactive monitoring was associated with remaining on the initial TNF inhibitor longer (hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.51–1.18; P = .24) and a much lower risk of cessation due to any detectable ADA (HR, 0.47; 95% CI, 0.27−0.79; P = .005).30
In a recent systematic review of real-world evidence on the long-term efficacy of IFX therapy in paediatric patients, the probability of still continuing IFX therapy was 83%–97% after one year, 67%–91% after 2 years and 61%–85% after 3 years. For ADL, there were fewer data, showing corresponding probabilities of 50%–85%, 50%–79% and 79% respectively.31 In our series, there was no loss of response or discontinuation of treatment.
Another aspect that we evaluated was the percentage of the duration of treatment that patients remained with the initial dose recommended by the manufacturer, and found that it was much higher in patients treated with ADL. One of the findings of Assa et al.14 was precisely that those under proactive monitoring required treatment intensification more frequently than those under reactive monitoring (87% vs 60%). A more detailed analysis showed that of the 33 patients that received ADA intensification, 18 did so due exclusively to concentrations of less than 5 μg/mL, while the remaining 15 also had levels under 5 μg/mL in addition to a faecal calprotectin level greater than 150 µg/g, a CRP greater than 0.5 mg/dL or a PCDAI of 10 or greater. In our opinion, a significant percentage of these patients may not have required intensification if they had been in proactive follow-up outside the framework of a clinical trial.
Numerous paediatric studies have provided cut-off points for mucosal healing, clinical remission, and histological healing at different time points in the follow-up of patients with IBD.22,32–34 In our study, the analysis of the cut-off points obtained with ROC curves to define clinical remission and mucosal healing did not evince the necessary predictive sensitivity or specificity, probably due to the sample size, although they were very close to those obtained in the study conducted by Kim et al.33 The adherence data reflected high compliance, as adherence was defined as a MPR of 85% or greater,35 which was achieved with ADL and almost achieved with IFX, and could be correlated to the results for the clinical outcomes.
One of the main limitations of our study is probably the sample size. Also, we estimated MH using the MINI, which, while offering a sensitivity of 84% and a specificity of 87% to detect MH, relies greatly on faecal calprotectin and may overestimate the actual health of the intestinal mucosa. We were also unable to analyse histological outcomes over time. Since MH is defined as endoscopic healing (according to different activity indices), it cannot be completely extrapolated from MINI scores, so our results regarding MH must be interpreted with caution. Another limitation was the lack of a control group. Our rates of clinical remission at 1, 2 and 3 years of follow-up are consistent with those reported by Kim et al.,33 but the frequencies of MH (Simple Endoscopic Score for Crohn Disease [SES-CD] vs MINI) were not, as they were much higher in the first two years. On the other hand, the method chosen to quantify therapeutic adherence may not correctly reflect the difference between ADL and IFX, also due to the difference size of the groups treated with each drug.
Every patient in which testing was performed had at least 2 of the DNA variants associated with a long-term response to TNF inhibitors described by Salvador et al.33 This pharmacogenetic aspect and the proactive measurement of drug levels probably contributed to the favourable outcomes obtained in our series. Fast laboratory turnaround times are essential for a proactive approach, as was the case in our study. This made it possible for patients to have samples collected 2 weeks before the consultation and receive the results on the day of their office visit. In the case of IFX, the rapid response later allowed contacting the family to schedule the new infusion. Furthermore, adherence in our cohort was high, consistent with data published by other authors.36
In conclusion, the proactive and serial monitoring of anti-TNF levels makes it possible for paediatric patients to maintain clinical remission and achieve mucosal healing in most cases through personalised optimisation of the required dosage and avoiding secondary loss of response. The measurement of drug concentrations is another possible tool for monitoring patients, although the correlation with mucosal healing or clinical remission may be low.
Conflict of interestThe authors declare that they have no conflict of interest.
We thank Jordi Bozzo, senior manager of scientific publications at Grifols, for his valuable contributions following the exhaustive review of the manuscript once the authors had finished drafting it.