Kawasaki disease (KD) is a multisystem vasculitis associated with coronary artery abnormalities. Infections could be a trigger of the inflammation. The main aim of this study was to describe the presence of infections in children with KD, and to analyse the clinical characteristics and the presence of coronary abnormalities in these cases.
Patients and methodsA retrospective study was performed within the Kawasaki Disease network (KAWA-RACE (2011-2016). An analysis was performed that included patients with positive microbiological findings (PMF) during the acute phase, as well as those with a previous recent infection (PRI) during the 4 weeks preceding KD diagnosis.
ResultsThe study included a total of 621 children with KD, with PMF being found in 101 (16.3%) patients, and a PRI in 107 (17.2%). Significantly less echocardiographic abnormalities were found in the group with a PRI, when compared to those without a PRI (23 vs. 35%, P=.01) and also a lower proportion of overall coronary artery lesions (16 vs. 25%, P=.054). No significant differences were found in the proportion of aneurysms in either of these groups (PRI or PMF) when compared to those without infection.
ConclusionsIn the present study, no differences were found in the incidence of coronary aneurysms in either of the groups, with or without PRI or PMF. Therefore, if KD is suspected, appropriate treatment should be started despite having a confirmed infection.
La enfermedad de Kawasaki (EK) es una vasculitis multisistémica asociada a lesiones en las arterias coronarias. Las infecciones podrían ser un desencadenante de la inflamación. Nuestro objetivo fue describir la presencia de infecciones en los niños con EK y analizar las características clínicas y la presencia de alteraciones coronarias en estos casos.
Pacientes y métodosAnálisis retrospectivo de los pacientes incluidos en la red KAWA-RACE entre 2011 y 2016. Se estudió tanto a los pacientes que tuvieron una identificación microbiológica confirmada (IMC) en el periodo agudo como a los que presentaron antecedente de infección previa reciente (IPR) las 4 semanas anteriores.
ResultadosSe incluyó a un total de 621 niños, de los cuales 101 (16,3%) tuvieron una IMC y 107 (17,2%) una IPR. Encontramos una significativa menor afectación ecocardiográfica en el grupo de IPR respecto a los niños sin infección previa (23 vs. 35%; p 0,01), con menor proporción no significativa de las alteraciones coronarias globales (16 vs. 25%; p 0,054). Sin embargo, no se detectaron diferencias en la proporción de aneurismas en ninguno de los 2 grupos (IMC o IPR) respecto al resto de los pacientes sin infecciones asociadas.
ConclusionesEn nuestro estudio no encontramos diferencias en la incidencia de aneurismas coronarios en niños con y sin IMC o IPR, por lo que ante la sospecha de EK debe iniciarse siempre tratamiento, aunque se tenga infección confirmada microbiológicamente.
Kawasaki disease (KD) is an acute self-limited vasculitis that affects small- and medium-size vessels and usually develops in early childhood. At present it is the most common cause of acquired heart disease in the spanish paediatric population.1,2 Its incidence in Europe is of approximately 8 per 100000 children under 5 years.3,4 Although the clinical manifestations, laboratory findings and epidemiological characteristics of the disease suggest an infectious trigger, the causative agent has yet to be identified.5,6 A few genome-wide association studies (GWAS) on KD have been published that identified several plausible candidate loci involved in inflammation, the immune response and cardiovascular abnormalities.2,7,8 Thus, a reasonably broad hypothesis is that KD is caused by an infectious agent yet to be identified that only causes disease in individuals with a genetic predisposition. Its rarity in the first months of life and in adults suggests that this is a pathogen that these collectives are immune to, with very young infants being protected by passive immunity through maternal antibodies.
Kawasaki disease is a clinical diagnosis that is determined based on the diagnostic criteria proposed by the American Heart Association (AHA).2 The presence of a previous or concurrent infection, especially with microbiological confirmation, can be considered an alternative diagnosis that rules out KD. This aspect is currently under debate. The aim of our study was to assess the presence of previous or concurrent infection in children with a diagnosis of KD based on data collected by the Spanish network for the study of this illness (KAWA-RACE), and its association with the presence of coronary artery abnormalities.
Patients and methodsIn 2015, a nationwide paediatric network was created in Spain for the purpose of researching KD (the KAWA-RACE network) with the support of the Sociedad Española de Infectología Pediátrica (Spanish Society of Paediatric Infectious Diseases [SEIP]), Sociedad Española de Reumatología Pediátrica (Spanish Society of Paediatric Rheumatology [SERPE]) and the Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas (Spanish Society of Paediatric Cardiology and Congenital Heart Diseases [SECPPC]). A total of 53 Spanish hospitals from all regions in the country participated, collecting epidemiological, clinical and treatment data on patients in an online database (REDcap).9 We conducted a retrospective study covering the 2011–2016 period, including patients aged less than 16 years that received a diagnosis of KD based on the AHA criteria10 in any of the participating hospitals. The study was approved by the Ethics Committee.
We used the following definitions:
- •
Recent previous infection (RPI): infection diagnosed in the 4 weeks preceding the diagnosis of KD, without need of a positive microbiological test to confirm the infection.
- •
Microbiologically-confirmed infection (MCI): infection diagnosed during the acute phase of KD with a positive microbiological test.
We defined echocardiographic abnormality as evidence of any type of cardiovascular involvement, including the presence of valvular insufficiency, pericarditis, signs of myocarditis or coronary artery abnormalities. Coronary artery abnormalities included the presence of hyperechoic lesions, dilatation or coronary aneurisms defined according to the AHA criteria.2
Statistical analysisWe performed the statistical analysis with the Statistical Package for the Social Sciences (SPSS) version 20.0. We have expressed discrete data as percentages and continuous data as mean and standard deviation (SD). We compared clinical and laboratory characteristics and the presence of coronary artery abnormalities in patients with and without RPI or MCI. The tests used for comparing clinical characteristics and laboratory findings were the Student t test, Mann–Whitney U test, χ2 test and Fisher exact test as appropriate. We defined statistical significance as a p-value of less than 0.05.
ResultsThe study included a total of 621 patients with KD. Sixty-three percent were male. The mean age at diagnosis was 2.8 years (SD, 2.4); 494 (79.5%) were aged less than 5 years. Of all cases, 437 were classified as complete KD (70.4%), 171 as incomplete KD (27.5%) and 13 as atypical (2.1%). There was evidence of coronary artery aneurisms in 60 cases (9.7%), which were persistent in 28 cases (4.5%).
Recent previous infectionsOf the 621 patients, 107 (17.2%) reported a RPI. These infections were most frequent in spring (38%; P=.023). We found no differences between patients with RPI and patients without in sex, age, type of KD, laboratory findings, total duration of fever or need of a second dose of intravenous immunoglobulin (Table 1). Of the 5 principal clinical features, we found differences in the presence of lymphadenopathy, which was more frequent in the RPI group (76% vs 64%; P=.01). We found a lower prevalence of echocardiographic abnormalities in the RPI group that was statistically significant (23 vs 35%; P=.01) as well as a lower proportion of coronary abnormalities, although this difference was not significant (16% vs 25%; P=.054). There were no differences in the presence of aneurysms.
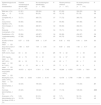
Epidemiological, clinical and laboratory characteristics of patients with Kawasaki disease based on the presence or absence of microbiologically confirmed infection and of a history of infection in the 4 weeks preceding diagnosis.
| Epidemiological and clinical characteristics | Positive microbiological identification (n=101) | No microbiological identification (n=520) | P | Recent previous infection (n=107) | No recent previous infection (n=514) | P |
|---|---|---|---|---|---|---|
| Male sex, n (%) | 61 (61) | 330 (64) | .62 | 67 (63) | 324 (63) | .54 |
| Age<12 months | 15 (16) | 88 (18) | .65 | 16 (16) | 87 (18) | .68 |
| Complete KD, n (%) | 72 (71) | 365 (70) | .97 | 77 (72) | 360 (70) | .64 |
| Conjunctival injection, n (%) | 83 (83) | 444 (86) | .37 | 90 (85) | 437 (86) | .76 |
| Oral changes, n (%) | 93 (93) | 468 (91) | .52 | 99 (93) | 462 (91) | .41 |
| Extremity involvement, n (%) | 70 (71) | 372 (74) | .54 | 75 (71) | 367 (74) | .65 |
| Exanthema, n (%) | 83 (84) | 443 (87) | .34 | 92 (89) | 434 (87) | .58 |
| Lymphadenopathy, n (%) | 67 (68) | 332 (65) | .65 | 80 (76) | 319 (64) | .01 |
| Duration of fever (days), mean±SD | 8.57±3.92 | 8.38±3, 67 | .64 | 8.35±4.02 | 8.43±3.64 | .83 |
| Days from onset of fever to IVIG, mean±SD | 7.88±6.87 | 7.04±3.84 | .10 | 6.69±3.02 | 7.30±4.77 | .23 |
| ESR (mm/h), mean ± | 83±34 | 72±34 | .01 | 78±32 | 73±35 | .23 |
| CRP (mg/dL), mean±SD | 46±70 | 35±61 | .12 | 34±57 | 37±64 | .59 |
| AST (IU/L), mean±SD | 88±18 | 75±6 | .42 | 63±7 | 80±7 | .20 |
| ALT (IU/L), mean±SD | 102±13 | 84±6 | .20 | 71±9 | 80±6 | .2 |
| White blood cell count (cells ×109/L), mean±SD | 18.073±6.210 | 17.911±7.745 | .84 | 18.298±6.553 | 17.870±7.631 | .63 |
| Neutrophil count, (cells ×109/L), mean±SD | 11.950±5.005 | 11.918±6.141 | .96 | 12.478±5.796 | 11.808±5.955 | .35 |
| ECHO abnormalities, n (%) | 32 (34) | 165 (33) | .85 | 24 (23) | 173 (35) | .01 |
| Coronary abnormalities, n (%) | 20 (20) | 123 (24) | .40 | 17 (16) | 126 (25) | .054 |
| Aneurysm, n (%) | 9 (9) | 50 (10) | .82 | 7 (7) | 52 (10) | .25 |
| Administration of a 2nd dose of IVIG, n (%) | 19 (19) | 79 (16) | .49 | 19 (19) | 79 (16) | .46 |
Statistically significant differences are presented in boldface.
ALT, alanine transaminase; AST, aspartate transaminase; CRP, C-reactive protein; ECHO, echocardiography; ESR, erythrocyte sedimentation rate; IVIG, intravenous immunoglobulin; SD, standard deviation.
The RPI documented most frequently were: ear-nose-throat (ENT) involvement, mainly pharyngitis (n=65; 61%), gastroenteritis (n=12; 11%), acute otitis media (n=7; 7%), urinary tract infection (n=6; 6%), scarlatiniform rash (n=5; 5%) and skin and soft tissue infections (n=3; 3%). Patients whose previous infection involved ENT involvement had coronary artery aneurysms in 10.5% of cases, a proportion that was higher compared to the rest of patients with a RPI (7%; P=.051).
Microbiologically confirmed infectionsWe identified 101 cases (16.3%) of patients with a MCI; 9 of them had several infections and 4 presented with viral coinfection (Table 2). Microbiologically confirmed infections were most frequent in spring (34% of the total; P=.07). Patients with MCI did not differ from patients with no microbiological confirmation of infection in sex, age, type of KD, clinical diagnostic criteria, total duration of fever or need of a second dose of intravenous immunoglobulin (Table 1). We found significantly higher erythrocyte sedimentation rates in the MCI group (83 vs 72mm/h). We did not find differences in the frequency of echocardiographic abnormalities overall or in the frequency of coronary artery abnormalities or aneurysms.
Positive microbiological results obtained in patients with Kawasaki disease (KAWA-RACE network, 2011–2016).
| Number of tests | Positive tests | Distribution of results | |
|---|---|---|---|
| Viral testing in nasopharyngeal aspirate samplea | 245 | 35 | Adenovirus (9), influenza virus (9), rhinovirus (7), RSV (4), herpes simplex virus (4), parainfluenza virus (2), coronavirus (1), |
| Bacterial testing in throat swab sample | 266 | 34 | SGA (29), Staphylococcus aureus (1), Pseudomonas aeruginosa (1), Haemophilus influenzae (1), Kingella kingae (1), other streptococci (1) |
| Urine culture | 273 | 16 | Escherichia coli (10), Proteus mirabilis (3), Enterococcus faecalis (2), Morganella morganii (1) |
| Stool culture | 109 | 14 | Rotavirus (9), adenovirus (3), dysbacteriosis (2) |
| Serologic testingb | – | 7 | Mycoplasma pneumoniae (2), parvovirus (3), EBV (2), CMV (2), Chlamydia pneumoniae (1) |
| Blood culture | 438 | 1 | Staphylococcus aureus and Streptococcus pneumoniae in a single patient |
| Lumbar puncture | 29 | 1 | Enterovirus |
| Other | – | 2 | Skin secretion sample for testing of Staphylococcus aureus (1), positive CMV PCR in urine (1) |
CMV, cytomegalovirus; EBV, Epstein-Barr virus; GAS, group A streptococcus; PCR, polymerase chain reaction; RSV, respiratory syncytial virus.
Our study evinces that recent or concurrent infections are common in KD and do not decrease the risk of developing coronary artery aneurysms. The presence of a RPI has also been described in the literature.5,6,11 Weng et al. conducted a population-based study where they followed up 285636 children with infection by enterovirus and 285636 children without this infection, and found that the incidence of KD was 54% greater in the infected group compared to the group without infection.5
The clinical manifestations of many of the most frequent infections in the paediatric population overlap the clinical features used to define KD, which complicates its diagnosis.2 In our case series, we found 65 patients (10.5%) with a history of ENT infection and 6 patients (1%) with a scarlatiniform rash, both infections whose manifestations overlap with the principal clinical features of KD. There were also 12 patients with gastroenteritis (2%), and previous studies in the literature have reported that KD may present with symptoms compatible with gastroenteritis,2,3 including a study conducted in Spain by Sánchez-Manubens et al that found them in as many as 24% of the patients.3
Historically, there has been concern that KD may be overdiagnosed.12 In our study we found a lower incidence of cardiac complications in the group of patients with a history of RPI, which may suggest that KD is also overdiagnosed in Spain,2 but we did not find differences in the presence of aneurysms.
In our cohort, the proportion of coronary artery involvement and more specifically of coronary artery aneurysms (7%–10%) was high compared to Asian case series in Japan (0.91%), Korea (1.7%) and Shanghai (1.1%), lower compared to the frequency reported in the United States (13%) and some European countries, such as the Netherlands (13.5%) or Germany (17%), but similar to the prevalence reported in case series of other neighbouring countries, such as Portugal (8.5%).8–14 However, Coon et al.15 recently published data for 48 hospitals in the United States and found that the frequency of diagnosis of non-severe coronary artery abnormalities in patients with KD has nearly doubled in the past 15 years, while there have been no significant changes in the proportion of cardiac complications. The same phenomenon of overdiagnosis might affect Europe, a situation that demands an analysis of which coronary artery lesions are clinically relevant with the aim of maximising benefits and minimising harm in these children.
The fact that lymphadenopathy was more frequent in the RPI group and that this feature is a criterion for the diagnosis of KD could contribute to the potential overdiagnosis of the disease in patients that do not actually have it. Thus, our findings evince that the diagnosis of KD continues to pose a significant challenge. Considering the risk of cardiac complications associated with not treating a true case of KD, we believe that utmost caution and rigour should be exercised when advising against treatment, especially in the groups at highest risk, which include infants and severely ill patients.
When it comes to the presence of concurrent infections, many studies in the literature have pointed at different viruses as triggers of KD, including coxsackievirus, parainfluenza virus, respiratory syncytial virus, metapneumovirus, chikungunya virus, cytomegalovirus and enterovirus, among others.5,6,11 Some studies have reported positive detection of one or more respiratory viruses by PCR in up to half of the patients with KD, although their pathogenic role is not clear.11 Chang et al selected 226 children with KD and 226 healthy children and performed PCR tests and cultures for detection of respiratory viruses in respiratory secretions. They found viruses in 50.4% of the ill children compared to 16.4% of healthy children (P<.001) and concluded that viral infections are associated with KD.4
On the other hand, we cannot overlook the nature of viral infections and the possibility that an individual remain an asymptomatic carrier for a variable period of time following a viral infection. One such example is infection by adenovirus, whose symptoms overlap with those of KD and which happens to cause significant elevation of acute phase reactants in laboratory tests, as we observed in patients in our series. Jaggi et al.16 reported detection of adenovirus in the throat of patients with complete and incomplete KD and in healthy individuals, and found that in patients with complete KD, the viral load was significantly lower compared to patients with incomplete KD, which led them to conclude that a positive test result for adenovirus should be interpreted with caution, as it could indicate a current infection by adenovirus in a patient that meets the criteria for KD or that a patient with KD is also an asymptomatic carrier of adenovirus.
Even if viruses are isolated from patients during the acute phase of KD, previous data have shown no differences in the incidence of coronary artery abnormalities in these patients compared to those that do not have viral infection, as was the case in our cohort.6 In our study, 35 patients (5.6%) tested positive for respiratory viruses, a significantly lower figure, but it must be taken into account that nasopharyngeal aspirate samples were obtained for viral detection in only 245 patients (39%), and that not all hospitals tested patients by means of respiratory virus PCR panels. The retrospective nature of our study and the absence of a shared protocol for microbiological diagnosis constitute the main limitations of this study. To establish the actual incidence of infection in patients with KD, the same microbiological tests would need to be performed in all patients, but there is not a standard protocol for testing and even the most recent guidelines of the AHA, from 2017, recommend individualised testing based on the judgement of the clinician and the symptoms of the patient.2
In short, based on our findings and those of previous studies, the presence of concurrent infection does not rule out the diagnosis of KD, which challenges the hypothesis that KD is overdiagnosed.
In conclusion, we did not find differences in the incidence of cardiac complications in patients with and without microbiological confirmation of infection, and therefore treatment should be initiated whenever there is suspicion of KD, whether or not infection is suspected or confirmed by a positive microbiological test. In our case series, recent previous infections were associated with a lower incidence of coronary abnormalities in patients with a KD diagnosis, although the incidence of coronary aneurysms was not lower in this group. It appears that high-quality prospective studies will be required to establish the role of infection in KD. We hope that the prospective recruitment already underway though the KAWA-RACE network, which we encourage every hospital in Spain to join, will contribute to elucidating this matter.
FundingThe KAWA-RACE group received a research grant from the Sociedad Española de Reumatología Pediátrica (SERPE) in the 2015 competition. These funds were devoted to the development of the working group and administrative expenditures.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all the researchers in the KAWA-RACE working group for their participation and contribution of patient information.
Please cite this article as: Fernández-Cooke E, Tascón AB, Antón-López J, Grasa Lozano CD, Sánchez-Manubens J, Calvo C, et al. Infecciones previas o coincidentes con la sospecha de enfermedad de Kawasaki ¿debemos cambiar nuestra actitud? An Pediatr (Barc). 2019;90:213–218.
Previous presentations: abstracts related to this study were submitted for participation in the Congress of the Asociación Española de Pediatría held in June 2018 in Zaragoza, Spain (accepted for oral communications) and the Annual Meeting of the European Society for Paediatric Infectious Diseases held in May 2018 in Malmö, Sweden (accepted for a poster presentation).