We propose to demonstrate that it is possible to implement a valid (diagnostic sensitivity for major cardiac malformations 90%), and universal (applied to over 90% of pregnant women), prenatal screening method for congenital heart defects.
Materials and methodsProspective study. A total of 12,478 pregnant women were evaluated between January 2008 and December 2010. Congenital heart diseases were screened using foetal extended basic echocardiography (cardiac ultrasound).
ResultsThe prevalence of birth defects in general and congenital heart disease was 2.5% (2.2–2.7%) and 0.9% (0.7–1%) respectively. Congenital heart disease had a higher rate of association with other structural abnormalities with 11.5% (5.6–17.4%), 21% for major congenital heart disease (9.9–32%), and chromosomal abnormalities of 15.9% (9.1–22.7%), with 32.6% for major congenital heart disease (19.8–45.3%). A foetal cardiac ultrasound assessment was performed on 99.2% of pregnant women. The foetal echocardiography is useful for the diagnosis of congenital heart disease in general, and major congenital heart disease, with a sensitivity of 42.8% (33.5–52.5%) and 90.4% (78.9–96.8%), respectively, and a specificity for both of 99.9% (99.8–99.9%).
ConclusionsIt is possible to perform a valid prenatal and universal screening of major congenital heart disease.
Nos proponemos demostrar que es posible la implantación de un cribado prenatal de cardiopatías congénitas de garantía (sensibilidad diagnóstica para malformaciones cardíacas mayores del 90%) y universal (aplicado a más del 90% de las gestantes).
Material y métodoEstudio prospectivo. Hemos valorado a 12.478 gestantes (enero del 2008-diciembre del 2010). Realizamos un cribado de cardiopatías congénitas aplicando una ecocardiografía foetal básica ampliada.
ResultadosLa prevalencia de los defectos congénitos en general y de las cardiopatías congénitas es del 2,5% (2,2-2,7%) y el 0,9% (0,7-1%), respectivamente. Las cardiopatías congénitas presentan una tasa de asociación a otras malformaciones estructurales del 11,5% (5,6-17,4%), 21% en caso de cardiopatía congénita mayor (9,9-32%) y a cromosomopatías del 15,9% (9,1-22,7%), 32,6% en caso de cardiopatía congénita mayor (19,8-45,3%). Hemos logrado realizar una valoración ecográfica cardiaca foetal al 99,2% de las gestantes. La ecocardiografía foetal presenta, para el diagnóstico de las cardiopatías congénitas en general y de las cardiopatías congénitas mayores, una sensibilidad 42,8% (33,5-52,5%) y el 90,4% (78,9-96,8%), respectivamente, y una especificidad para ambas del 99,9% (99,8-99,9%).
ConclusionesEs posible realizar un cribado prenatal de garantías y universal de las cardiopatías congénitas mayores.
The probability of a child being born with some type of congenital defect ranges from 2% to 4%.1 Congenital heart defects are the most prevalent birth defects (0.8–1%; 1 in 125 neonates).1,2 Their frequency in live births is between 5 and 7 times greater than the frequency of chromosomal abnormalities and between 3 and 4 times the frequency of neural tube defects.3,4 Over 50% of congenital heart defects are considered major malformations,4–6 with a global mortality that ranges from 25% to 35%.4,5,7 They cause between 20% to 30% of neonatal deaths, and over 50% of the deaths due to birth defects in children.4,5,7 They are also frequently associated with other malformations (in 20% of cases) and chromosomal abnormalities (between 20% and 40% of cases), so congenital heart defects have a high rate of perinatal and neonatal mortality.4–11 Although there are risk groups for congenital heart defects, 90% of them occur in low-risk pregnancies.12
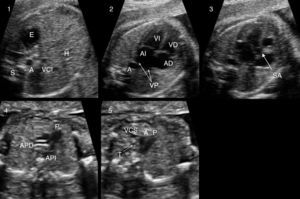
Thus, identification of congenital heart defects is one of the main goals of the morphology ultrasound examination (18–22 weeks). Still, the rate of detection of congenital heart defects by assessment of the four chambers of the foetal heart is inadequate.12–14 To improve results we propose performing the extended basic foetal cardiac examination proposed by Yagel (foetal abdomen, four-chamber view, great vessel outflow tracts, and thoracic three-vessel view; Fig. 1).15
Extended basic examination proposed by Yagel. Plane 1: abdominal view. Plane 2: four-chamber view. Plane 3: five-chamber view, aortic root. Plane 4: pulmonary artery bifurcation view. Plane 5: three vessel and trachea view. A: aorta; AD: right atrium; AI: left atrium; APD: right pulmonary artery; API: left pulmonary artery; E: stomach; H: livery; P: pulmonary artery; S: spine; SA: aortic aortic root; T: trachea; VCI: inferior vena cava; VCS: superior vena cava; VD: right ventricle; VI: left ventricle; VP: pulmonary veins.
Our aim was to demonstrate that a valid, universal screening system for congenital heart defects could be implemented by means of extended basic foetal echocardiography, achieving a detection rate above 90%.
Materials and methodsThe study ran for 3 years (January 2008–December 2010) and included a total of 12,478 gestations in our public health area, which has a population of 356,318.
We screened for structural malformations with a morphology ultrasound examination performed at 20 weeks (18–22 weeks) in the prenatal diagnosis unit of the Hospital Universitario de Valme. The examination lasted a minimum of 20min and was performed by one of three highly qualified sonographers (with over 5 years’ full-time experience in obstetric ultrasound) in compliance with the recommendations of the Sociedad Española de Obstetricia y Ginecología (Spanish Society of Obstetrics and Gynaecology)16 and the Royal College of Obstetricians and Gynaecologists17 for the performance of structural ultrasound examinations. The screening for congenital heart defects at 20 weeks of gestation involved an extended basic foetal heart examination15 of five transverse planes (Fig. 1). The ultrasound machines used for the examination were 1 Philips HDI 4000 system (Philips Medical Systems) and 1 GE E8 (General Electric).
The structural anomalies detected were classified as proposed by the Eurofetus study.13 Following the guidelines of the Eurocat group,14 we considered congenital heart defects major if they were likely to require surgical intervention because they result in altered function: endocardial cushion defect; ventricular septal defect (VSD) larger than 3mm; common ventricle; tricuspid atresia with or without VSD; tricuspid valve dysplasia or Ebstein's anomaly; severe pulmonary atresia or stenosis; hypoplastic left heart syndrome; severe aortic stenosis; tetralogy of Fallot; transposition of the great arteries; double outlet right ventricle; common arterial trunk; coarctation of the aorta or interrupted aortic arch; total anomalous pulmonary venous connection; heart neoplasm; or cardiomyopathy. We considered congenital heart defects minor if they were not likely to require surgical intervention because they were of little functional significance: patent ductus arteriosus; atrial septal defect; VSD smaller than 3mm; mild mitral, tricuspid, pulmonary, or aortic stenosis; absence of inferior vena cava with azygos continuation; and cardiac arrhythmia without a structurally normal heart.6
To complete the screening for congenital defects, a combined test was also used to screen for chromosomal abnormalities (pregnancy-associated plasma protein A, free beta subunit of human chorionic gonadotropin, nuchal translucency) between 11 and 13+6 weeks of gestation.18,19
If congenital heart disease was suspected, an evaluation was performed in the foetal medicine unit by staff subspecialised in foetal ultrasonography and paediatric cardiology to confirm the diagnosis, to provide subsequent postnatal counselling, and offer performance of invasive procedures. Pregnancies in which congenital heart defects were detected were followed up in the foetal medicine unit every 2–4 weeks, and care was transferred to a tertiary referral hospital if the diagnosed heart condition could require medical intervention by cardiac catheterisation or surgery in the first weeks of life.
All newborns were examined and monitored by the paediatrics team of the Hospital Universitario de Valme in the first 72h of life.
When congenital heart disease was suspected or diagnosed prenatally and the mother chose to proceed with the pregnancy, the paediatric cardiology unit performed a study in the first 48h of life that included a physical examination, measurement of blood pressure in arms and legs, an electrocardiogram, and a 2D/Doppler ultrasound scan. Likewise, all patients diagnosed with cardiopathies at a later stage in outpatient or inpatient services were also monitored. We also monitored readmissions to the paediatric unit due to various conditions up to 1 year of age, and evaluated the possibility of a congenital heart disease not previously diagnosed.
If the pregnancy was terminated or a neonate died for undetermined causes, a pathologist with specialised training and work experience in foetal malformations performed an autopsy of the remains to confirm the suspected prenatal diagnosis.
The criteria we chose to determine whether this examination could serve as a universal and valid screening for congenital heart defects to be included in the overall screening for congenital defects were: achieving a detection rate of congenital heart defects above 90%, and managing to perform it in more than 90% of the pregnancies.
Statistical analysisSample size: we calculated a sample size of 24 pregnancies with congenital heart disease and 18 with a major congenital heart defect for a sensitivity of 90% versus the 65% established in a screening for major congenital heart defects in a finite population of 12,500 pregnancies with a false positive rate greater than 5%, a prevalence of congenital heart defects of 1% and of major heart defects greater than 0.5%; for an α error of 5% and a power of 80% in a two-tailed test.
Sample sizes were calculated using the nQuery Advisor software, version 4.0. We performed the data analysis using the SPSS software, version 19.0 for Windows.20
ResultsIn the period under study there were a total of 12,478 pregnancies that resulted in 12,668 live births. Table 1 describes the obstetric and perinatal outcomes of the group under study.
Obstetric outcomes in Hospital Universitario de Valme between January 2008 and December 2010.
| Number of gestations | 12,478 |
| Number of twin gestations | 267 (2.14%) |
| Mean age of pregnant women in years | 30.03±5.3 (14–47) |
| Mean gestational age at delivery in weeks | 38.97±1.6 (24–42) |
| Preterm birth rate (a<37 weeks) (b<32 weeks) | a 7.1% b 1.2% |
| Caesarean delivery rate | 20.5% |
| Percentage of newborns with weight≤2500g | 7.4% |
| Percentage of newborns with weight≤1000g | 0.34% |
| Number of live births | 12,622 |
| Number of still births | 46 |
| Number of voluntary terminations of pregnancy | 78 |
The morphology ultrasound could not be performed in 59 pregnancies (0.47%) and had to be repeated in 412 (3.3%).
Some type of congenital defect was detected in 323 foetuses (2.5%; 2.2–2.7%), leading to termination of pregnancy in 78 cases (47 due to malformations and 31 due to chromosomal abnormalities). There were 35 cases of chromosomal abnormalities (prevalence, 0.2%) and 288 foetuses had some form of malformation, with a prevalence of 2.3% (2–2.6%). The most common malformations were heart defects (38.8%), followed by kidney defects (25.3%) and musculoskeletal defects (12.5%).
We were able to perform an extended basic foetal echocardiogram on 99.2% of the pregnant women (12,398 gestations). There were 112 cases of congenital heart defects, with a prevalence of 0.9% (0.7–1%), of which 46% (52 cases) were major heart defects (prevalence, 0.4%; 0.2–0.5%). The most frequent major congenital heart defect was VSD larger than 3mm (14 cases), followed by endocardial cushion defect (seven cases). There were 19 cases of congenital conotruncal cardiopathy (Table 2).
Prevalence and rate of prenatal ultrasound diagnosis of major and minor congenital heart defects.
| Total, N (%) | Diagnosed | Undiagnosed | |
| Major heart malformations | 52 (46%) | 47 (90.4%) | 5 (9.6%) |
| VSD>3mm | 14 | 13 | 1 |
| Endocardial cushion defect | 7 | 7 | 0 |
| Common ventricle | 0 | 0 | 0 |
| Tricuspid atresia with VSD | 0 | 0 | 0 |
| Tricuspid atresia without VSD | 1 | 1 | 0 |
| Tricuspid valve dysplasia | 1 | 1 | 0 |
| Ebstein's anomaly | 0 | 0 | 0 |
| Severe pulmonary stenosis, atresia | 2 | 2 | 0 |
| Hypoplastic left heart syndrome | 2 | 2 | 0 |
| Severe aortic stenosis | 0 | 0 | 0 |
| Tetralogy of Fallot | 3 | 3 | 0 |
| Transposition of the great arteries | 2 | 2 | 0 |
| Common arterial trunk | 6 | 6 | 0 |
| Double outlet right ventricle | 2 | 2 | 0 |
| Coarctation of the aorta | 6 | 3 | 3 |
| Interrupted aortic arch | 0 | 0 | 0 |
| Total anomalous pulmonary venous connection | 0 | 0 | 0 |
| Other (ectopia cordis, heart tumour, cardiomyopathy) | 4 | 3 | 1 |
| Complex heart defect:hypoplastic left heart+endocardial cushion defect, TGA+VSD+hypoplastic left heart | 2 | 2 | 0 |
| Minor heart defects | 60 (54%) | 1 (1.7%) | 59 (98.3%) |
| Atrial septal defect | 8 | 0 | 8 |
| VSD<3mm | 41 | 1 | 41 |
| Mild pulmonary stenosis | 8 | 0 | 8 |
| Other (mild stenoses or valve regurgitations) | 2 | 0 | 2 |
| Total | 112 (100%) | 48 (42.8%) | 64 (57.1%) |
IVC: inferior vena cava; TGA: transposition of the great arteries; VSD: ventricular septal defect.
Congenital heart defects were associated with other structural abnormalities in 11.5% (5.6–17.4%) of cases, 21% of which corresponded to major congenital heart defects (9.9–32%); and were associated to chromosomopathies in 15.9% of cases (9.1–22.7%), a percentage that rose to 32.6% (19.8–45.3%) in cases of major congenital heart defects (Table 3).
Prevalence of chromosomal abnormalities and extracardiac malformations associated with congenital heart defects (major and minor congenital heart defects).
| Total number of congenital heart defects (cases, %) | Major heart defects (cases, %) | Minor heart defects (cases, %) | |
| Total number | 112 (100%) | 52 (46%) | 60 (54%) |
| Association with other malformations | 13 (11.5%) | 11(21%) | 2(3.2%) |
| System involved in associated malformation | CNA 6, renal 3, musculoskeletal 2, other 4 | CNS 6, renal 3, other 4 | Musculoskeletal 2 |
| Association with chromosomal abnormalities | 18 (15.9%) | 17 (32.6%) | 1 (1.6%) |
| Associated chromosomal disorders | 11 T21, 3 T18, 3 T13, 1 69 XXX | 10 T21, 3 T18, 3 T13, 1 69 XXX | 1 T21 |
CNS: central nervous system.
Using the extended basic foetal heart examination we have achieved a sensitivity of 42.8% (33.5–52.5%) in the prenatal diagnosis of congenital heart defects overall, and of 90.4% (78.9–96.8%) in the diagnosis of major congenital heart defects; with a specificity of 99.9% (99.8–99.9%) for both (Table 4). A prenatal diagnosis was not made in three cases of coarctation of the aorta, one case of VSD greater than 3mm, and one case of cardiomyopathy. The false positive rate in the diagnosis of congenital heart defects was 0.07% (0.03–0.09%) of the total number of gestations (nine cases: six of VSD, one of ASD, one of suspected coarctation of the aorta, and one of anomalous pulmonary venous connection).
Sensitivity, specificity, positive and negative predictive values of extended basic foetal echocardiography for the prenatal diagnosis of congenital heart defects overall and major congenital heart defects in particular.
| N | Sen | Spe | PV+ | PV− | |
| All congenital heart defects | 112 | 42.8%(33.5–52.5%)(48/112) | 99.9%(99.8–99.9%)(12,277/12,286) | 84.2%(74.7–93.6)(48/57) | 99.4%(99.1–99.5%) (12,277/12,341) |
| Major congenital heart defects | 52 | 90.4%(78.9–96.8%)(47/52) | 99.9%(99.8–99.9%)(12,344/12,346) | 95.9%(89.9–99.9%)(47/49) | 99.9%(99.8–99.9%)(12,344/12,349) |
Total number of extended basic echocardiography studies: 12,398 (31 voluntary terminations of pregnancy before 20 weeks and 59 cases dropped from study).
N: number; PV+: positive predictive value; PV−: negative predictive value; Sen: sensitivity; Spe: specificity.
Of the total of 112 prenatal diagnoses of congenital heart defect, 84 resulted in a live birth (66.9–83%), 25 (22.3%; 14.6–30%) in voluntary termination of pregnancy, and three in intrauterine death (Table 5).
Evaluation of pregnancies with congenital heart defects.
| Total CHDs/major CHDs | Voluntary terminations of pregnancy in relation to the total number of CHDs/in relation to the total number of major CHDs | Intrauterine deaths in relation to the total number of CHDs/in relation to the total number of major CHDs | Live NBs with CHD/Live NBs with major CHD | |
| N (%) | 112 (100)/52 (46) | 25 (22.3)/25/52 (48) | 3 (2.6)/3 (5.7) | 84 (75)/24 (46) |
| Description | – | CHD associated with a chromosomal abnormality or another malformation 17 (68%) | Isolated CHD 8 (32%)(4 hypoplastic ventricle, 2 truncus arteriosus, 1 ECD, 1 rhabdomyoma-tuberous sclerosis | 1 complex CHD, 1 Fallot, 1 RV hypoplasia associated to CNS malformation |
CHD: congenital heart defect; CNS: central nervous system; ECD: endocardial cushion defect; NB: newborn; RV: right ventricle.
The prevalence of congenital malformations in our population (2.5%) is similar to those reported for low-risk populations by various congenital defect registries.1 The most prevalent malformation were heart defects (0.9%), with an incidence of major congenital heart defect of 0.4%, figures that are consistent with those reported by other studies of congenital malformations in low-risk populations.2,6,7,13,14,21
The detection of major congenital heart defects was a priority for the prenatal congenital malformation screening programme, as these are the most prevalent structural malformations1,2 and have high perinatal morbidity and mortality rates.4,5,7 Likewise, detection of minor congenital heart defects is not included in the objectives of prenatal screening programmes because they have low perinatal morbidity and mortality.14
The factors that most influence the diagnostic capability of foetal echocardiography for congenital heart defects are the population of pregnant women undergoing echocardiography; type of examination and time of gestation at which it is performed; type of congenital heart defect; and experience of the ultrasonographer.4,5,10
Although there are risk groups for congenital heart disease (family history of congenital heart disease; maternal disease during pregnancy: diabetes, lupus, exposure to teratogenic or pharmacological agents; foetal disease: intrauterine growth restriction, oligohydramnios, polyhydramnios, etc.), selective performance of foetal echocardiography in these patients fails to detect most congenital heart malformations, as 90% of them occur in the low-risk population.4,5,10
Originally it was believed that a four-chamber view of the foetal heart would suffice to detect most major congenital heart defects before birth,5 but the universal implementation of this method in the low-risk population has not achieved good results (the detection rate for major congenital heart defects has not reached past 25%)22,23; so it has been suggested that an assessment of the great vessels is added to the heart examination15 for an extended basic foetal echocardiogram, a method with which some research groups have achieved detection rates of 60% to 80%6,8 for major congenital heart malformations.
While some major congenital heart defects can be identified in the first trimester, it is in the second trimester (18–22 weeks of gestation) that the heart structures can be viewed properly for their evaluation, and the detection rate increases over 25% between the first and second trimesters of gestation.6
Other factors at play in the capability to diagnose congenital heart defects are the type of congenital defect and the experience of the sonographer. There are congenital heart defects such as hypoplastic left heart syndrome, common ventricle, or Ebstein's anomaly for which the detection rate of prenatal echocardiography is greater than 50%, but for others such as transposition of the great arteries, tetralogy of Fallot, common arterial trunk, total anomalous pulmonary venous connection, VSD and ASD the detection rate is below 20%.14,24 Differences in detection rates of over 30% have also been observed between groups with specialised education in foetal echocardiography or that have received specific training in it, and groups with no specialised training.24,25
Thus, at present the proposed method for screening for heart defects is performance of an extended basic examination on all pregnant women at 18–22 weeks of gestation by staff specifically trained in foetal echocardiography.4,5,15 We followed these recommendations and performed an extended basic scan of five sequential planes on all our pregnant patients at between 18 and 22 weeks of gestation. Ultrasound scans were carried out in the prenatal diagnosis unit by staff with specific training in foetal echocardiography and more than 5 years’ experience in prenatal diagnosis. We achieved a prenatal detection rate of congenital heart defects greater than 90.4%, as we had set out to do, and demonstrated that most major congenital heart defects can be detected before birth. We achieved a very low prenatal detection rate for minor congenital heart defects, but in addition to the scarce impact on postnatal life of these defects, the diagnostic capability of prenatal ultrasound for these conditions is very limited (with diagnostic rates below 10%).14,24,26
Another relevant aspect of congenital heart defects in prenatal life is their frequent association with chromosomal abnormalities and other congenital malformations (30%).5,6,21,24,27 Such associations raise the mortality of congenital heart defects to up to 70%.6,11,25 In our series, the rate of association with chromosomal abnormalities or extracardiac malformations was lower (11.5%), although this rate rose to 21% for major congenital heart defects. Consequently, when a congenital heart defect is detected, an appropriate examination by ultrasound of the other foetal structures and a foetal karyotype analysis should be performed,18,19 as the main prognostic factor for major congenital heart defects in intrauterine life is its association or lack thereof with chromosomal abnormalities or other structural defects.6,11,25
The benefits of prenatal detection of congenital heart defects include avoiding the transfer of patients with major congenital heart defects; planning the birth and early treatment; reducing the need for mechanical ventilation, vasopressor or inotropic agents, or prostaglandins; and even reduce the morbidity and mortality of some congenital heart defects (transposition of the great arteries, coarctation of the aorta, and hypoplastic left heart syndrome).26–31 In this regard, there is evidence of reduced neonatal morbidity and mortality when neonates with major congenital heart defects requiring intensive care after birth (transposition of the great arteries, hypoplastic left heart syndrome) are referred in utero for delivery in a tertiary care hospital (up to 40% of immediate neonatal death in newborns requiring transfer to a tertiary hospital are due to the presence of a congenital heart defect); similarly, it has been observed that adequate planning of referrals and specialised transport improves morbidity in these patients (10–20% of newborns transferred by means of conventional transport develop temperature instability, hypoglycaemia, or hypo/hypercapnea). The appropriate management of referrals and transport in newborns with heart defects requires an adequate detection rate of major congenital heart defects, and we have established that detection can be achieved in 90% of these cases.26,32–35
Furthermore, the prenatal diagnosis of major congenital heart defects provides valuable information for parents for making decisions about how to manage the pregnancy, and to prepare them mentally.36,37 It is important in these cases that parents are offered counselling from a multidisciplinary approach, with liaison between the paediatric cardiology and the prenatal diagnosis units.36,37 Thus, the prenatal detection of major congenital heart defects has changed the prevalence at birth of different types of congenital heart disease, and a decrease of 15% in the prevalence at birth of major congenital heart defects has been reported.38 In our series, we observed a 22.3% reduction in the birth of foetuses with congenital heart disease, and voluntary termination of pregnancy occurred mainly in cases of very severe congenital heart defects and heart defects associated to chromosomal abnormalities or other congenital malformations.
The false positive rate for prenatal diagnosis of foetal malformations using ultrasound ranges between 0.5% and 0.7%.39 The most common false-positive diagnosis are pyelectasis, ventriculomegaly and abdominal cysts. Of all false positives, 10% are for heart defects, usually for septal defects, mild or moderate stenoses, and coarctations of the aorta, as many of these may be part of normal evolution during foetal life or their prenatal diagnosis is only suspected (as is the case of coarctation of the aorta).40 In our study, the false positive rate for detection of congenital heart defects was 0.07% (8% of all heart defects) which we find quite acceptable, as it causes few cases of anxiety to parents.
One limitation of our study is that postnatal identification of heart defects is restricted to the first year of life, and that it is based on signs and symptoms and not on the routine echocardiographic study of every newborn, which means that our study may have missed some minor congenital heart defects or defects with delayed onset of symptoms. There were also cases of spontaneous miscarriage in which the anatomical pathology examination offered limited information, which may also have led to cases of congenital heart disease that were not included in our case count. It is difficult to control such errors in prenatal series, and the various registries of foetal malformations need to take them into consideration. Such errors often lead to reporting differing prevalences of congenital malformations.1,38
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sainza JA, Zuritaa MJ, Guillenb I, Borreroa C, García-Mejidoa J, Almeidac C, et al. Cribado prenatal de cardiopatías congénitas en población de bajo riesgo de defectos congénitos. Una realidad en la actualidad. An Pediatr (Barc). 2015;82:27–34.