The aim of this study is to identify predictive factors of bacterial contamination in positive blood cultures (BC) collected in an Emergency Department.
Patients and methodsA prospective, observational and analytical study was conducted on febrile children aged one to 36 months, who had no risk factors of bacterial infection, and had a BC collected in the Emergency Department between November 2011 and October 2013 in which bacterial growth was detected. The potential BC contamination predicting factors analysed were: maximum temperature, time to positivity, initial Gram stain result, white blood cell count, absolute neutrophil count, band count, and C-reactive protein (CRP).
ResultsBacteria grew in 169 BC. Thirty (17.8%) were finally considered true positives and 139 (82.2%) false positives. All potential BC contamination predicting factors analysed, except maximum temperature, showed significant differences between true positives and false positives. CRP value, time to positivity, and initial Gram stain result are the best predictors of false positives in BC. The positive predictive values of a CRP value≤30mg/L, BC time to positivity≥16h, and initial Gram stain suggestive of a contaminant in predicting a FP, are 95.1, 96.9 and 97.5%, respectively. When all three conditions are applied, their positive predictive value is 100%. Four (8.3%) patients with a false positive BC and discharged to home were revaluated in the Emergency Department.
ConclusionsThe majority of BC obtained in the Emergency Department that showed positive were finally considered false positives. Initial Gram stain, time to positivity, and CRP results are valuable diagnostic tests in distinguishing between true positives and false positives in BC. The early detection of false positives will allow minimising their negative consequences.
El objetivo del estudio es identificar factores predictores de contaminación ante un hemocultivo (HC) con crecimiento bacteriano realizado en un servicio de Urgencias.
Pacientes y métodosEstudio prospectivo, observacional-analítico. Se incluyen los pacientes de uno a 36 meses, febriles, sin factores de riesgo para bacteriemia, con un HC realizado en el Servicio de Urgencias entre noviembre de 2011 y octubre de 2013 en el que se observa crecimiento bacteriano. Se analizan como posibles factores predictores de contaminación: temperatura máxima, tiempo de positividad, resultado inicial de la tinción de Gram, leucocitos totales, neutrófilos totales, neutrófilos inmaduros y proteína C reactiva (PCR).
ResultadosSe incluyen 169 casos. El crecimiento bacteriano del HC se considera significativo (positivo) en 30 (17,8%), y contaminado en 139 (82,2%). Todos los factores predictores analizados, a excepción de la temperatura, presentan diferencias estadísticamente significativas entre los 2 grupos. Los 3 mejores predictores de contaminación son la PCR, el tiempo de positividad y el resultado inicial de la tinción de Gram. El valour predictivo positivo de una PCR≤30mg/L, un tiempo de positividad≥16h y una tinción de Gram con morfología bacteriana considerada como probable contaminación es del 95,1, 96,9 y 97,5%, respectivamente; el valour predictivo positivo es del 100% para la combinación de los 3 factores. Se reevalúan el 8,3% de los pacientes con un HC contaminado dados de alta inicialmente a domicilio.
ConclusionesLa mayoría de HC con crecimiento bacteriano son finalmente considerados contaminados. El resultado inicial de la tinción de Gram, el tiempo de positividad y el valour de la PCR permiten identificarlos precozmente. Su pronta detección permitirá reducir las repercusiones negativas derivadas de los mismos.
Infectious disease is the main reason for care seeking in paediatric Emergency Departments (EDs). At times, the evaluation of these patients requires microbiological studies, and blood culture (BC) is among the tests most frequently ordered.1 Fever without a source in infants at risk of potentially severe bacterial infection is the main reason for requesting BCs, and they are also frequently performed in febrile patients with immunosuppressive conditions and in children with bacterial infections requiring hospital admission for parenteral antibiotic treatment before starting its administration.2
Following the introduction of routine vaccination with the seven-valent pneumococcal conjugate vaccine (PCV), the rate of occult bacteraemia (OB) has decreased drastically to values below 0.5–1%.3–7 It is expected that the universal administration of the 13-valent PCV will lower this rate even further in reducing other forms of invasive pneumococcal disease. In this new scenario, most BCs performed in EDs that show bacterial growth turn out to have been contaminated.3,5,7 Furthermore, the continuous-monitoring BC systems that are currently available can detect bacterial growth in the first 24h in most instances, while in the past detection occurred considerably later.8 This decrease in detection time has a significant impact on the therapeutic approach to these patients, as in this reduced timeframe most of them are still febrile when they return for reevaluation, which may lead to a more aggressive management. In this emerging reality, the early identification of these patients is essential to avoid the unnecessary use of healthcare resources that often ensues, especially in the case of patients discharged to their homes (return visits, additional diagnostic testing, hospital admission and/or initiation of antibiotic treatment, etc.),3,9 thus minimising the anxiety caused to patients and their families. While different variables have been proposed as possible predictive factors for contamination in BCs with bacterial growth, such as time to positivity and Gram stain results,3,9–11 we are not aware of any studies on the subject published after the changes discussed above that have occurred in recent years.
The primary objective of this study was to identify predictive factors for contamination in BCs with detected bacterial growth performed in the ED in febrile patients 1–36 months of age, and the secondary objective was to analyse the impact of BCs found to be contaminated in patients discharged to their homes.
Patients and methodsWe conducted a prospective observational and analytical study in a tertiary referral maternity and children's hospital with 275 paediatric beds that serves a catchment population of 1800000 inhabitants and receives a mean of 95000 paediatric emergency visits a year.
Using the electronic database of the hospital's laboratory, we selected the medical records of patients 1–36 months of age with a BC ordered by the ED in which bacterial growth was detected. The period under study was 2 years (November 2011–October 2013). We included febrile patients with no risk factors for bacteraemia. The study included patients with fever without a source and also patients in which the cause of the fever was identified in the ED. Fever was defined as an axillary temperature of 38°C or higher at home or at the ED, and the risk factors for bacteraemia included the presence of an immunosuppressive condition; having an indwelling catheter, prosthetic valve or cerebrospinal fluid shunt; and/or a history of hospital admission or aggressive diagnostic or therapeutic procedures in the week preceding the ED visit.
Blood extractions for BC in the ED were ordered based on the judgement of the doctor in charge, in compliance with current protocols.12
Blood culture specimens were collected by the nursing staff of the ED by percutaneous venepuncture.13 The blood volume recommended for the type of BC bottle used is 4mL. However, minimum volumes have been established depending on the age of the patient (1mL for children younger than 3 months, 2mL for children 3 months to 3 years of age). After the blood was drawn, it was inoculated in a paediatric bottle (BacT/ALERT® PF) for aerobic culture and transported to the microbiology department for its immediate processing in the automated BacT/ALERT® incubation system (Biomérieux, Durham, USA) for a maximum period of 5 days. All BCs with bacterial growth were subjected to Gram staining and subcultured in different culture media (blood agar, chocolate agar) at 37°C. Whenever the microbiologist detected bacterial growth in a BC and, based on the patient's chart, considered it was likely to be pathogenic, the paediatrician in charge was notified, who in turn called the patient, if he or she had been discharged home, to schedule a reevaluation at the ED within a short timeframe.
Variables analysedWe collected data for the following clinical and epidemiological variables by means of a previously designed questionnaire: age and sex, pneumococcal vaccination status, previous antibiotic treatment, characteristics of fever, final diagnosis, prescription of antibiotic therapy and disposition of the patient after the initial visit to the ED. For patients discharged home that had a contaminated BC we gathered additional information on return visits to the ED, further diagnostic tests, hospital admission and/or initiation or change of antibiotic treatment. We also collected laboratory data (leukocytes, total neutrophils, band cells and C-reactive protein [CRP]) and microbiological data (Gram stain result, time to positivity of BC, final result of BC, isolated microorganism and concordance with the initial Gram stain result) for every patient.
We analysed the following variables as potential predictive factors for contamination in the event of a BC with bacterial growth: maximum body temperature, time to positivity of the BC, detection of a bacterial morphology suggestive of contamination in the Gram stain, and the following laboratory parameters: total leukocytes, total neutrophils, band cells and CRP. We analysed these variables for the entire sample and for the subset of patients at high risk of OB, which consisted of patients 2–36 months of age with fever without source and in good general condition discharged home from the initial ED visit.
Definitions- -
Time to positivity of the BC: time elapsed from the beginning of incubation to the detection of bacterial growth in the BC.
- -
Positive BC: isolation of a bacterium that is usually pathogenic in the paediatric age group, which typically corresponds to: Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitidis, Enterococcus, enterobacteria (Escherichia coli, Klebsiella spp, Enterobacter spp., etc.) group A and B Streptococcus, Staphylococcus aureus, Salmonella spp., and Pseudomonas aeruginosa.
- -
Contaminated BC: isolation of a bacterium that is usually a contaminant in a patient with no risk factors: coagulase-negative Staphylococcus spp., Micrococcus, Clostridium spp., Gram-positive bacilli, nonpathogenic Neisseria spp., Streptococcus viridans, nonhaemolytic Streptococcus, and diphtheroids.
- -
Gram result suggestive of pathogen: Gram stain identification of Gram-positive cocci in pairs or chains, Gram-negative rods, Gram-negative coccobacilli or Gram-negative diplococci.
- -
Gram result suggestive of contaminant: Gram stain identification of Gram-positive cocci in clusters or Gram-positive bacilli.
We analysed the collected data with the IBM® SPSS® statistics software for Windows®, version 20.0 (IBM Corp, Armonk, NY, USA), running tests to analyse their distribution (Kolmogorov–Smirnov) and compare quantitative data (Student's t, Mann–Whitney U) and qualitative data (chi square, contingency table, Fisher's exact test). We calculated 95% confidence intervals by the Wilson method. We set the level of statistical significance at P<.05.
To analyse predictive factors for contamination we excluded cases with incomplete data. We performed receiver operating characteristics (ROC) analysis for continuous variables in which we had observed significant differences, calculating the optimal cut-off point. We calculated the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPP) for the three predictive factors of contamination with the highest statistical significance.
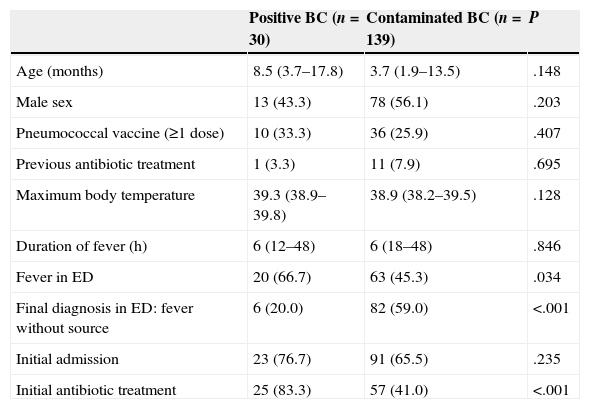
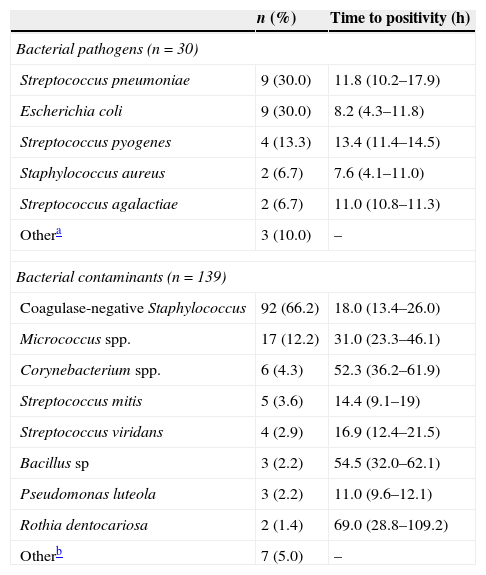
ResultsWe analysed the data for 169 BCs with bacterial growth, of which 30 (17.8%) were true positives and 139 (82.2%) were contaminated. Table 1 shows the clinical and epidemiological characteristics of both groups. Thirty-three (19.5%) BCs corresponded to patients 2–36 months of age with fever without a source and in good general condition that were discharged home. The bacteria isolated most frequently in positive BCs were S. pneumoniae (nine cases; 30.0%) and E. coli (nine cases; 30.0%), with a mean time to growth of 11.8 and 8.2h, respectively. Coagulase-negative staphylococci (92 cases; 66.2%) were the most frequent contaminants of BCs, and had a mean time to growth of 18.0h. Table 2 shows the distribution and time to positivity of the identified bacterial pathogens and contaminants. The Gram stain results were suggestive of a contaminant in 124 (73.4%) BCs, suggestive of a pathogen in 44 (26.0%), and negative in the rest (0.6%), with concordance between the initial Gram stain result and the final identification of the isolate in 167 (98.8%) cases.
Clinical and epidemiological characteristics of the 169 patients by final blood culture result.
| Positive BC (n=30) | Contaminated BC (n=139) | P | |
|---|---|---|---|
| Age (months) | 8.5 (3.7–17.8) | 3.7 (1.9–13.5) | .148 |
| Male sex | 13 (43.3) | 78 (56.1) | .203 |
| Pneumococcal vaccine (≥1 dose) | 10 (33.3) | 36 (25.9) | .407 |
| Previous antibiotic treatment | 1 (3.3) | 11 (7.9) | .695 |
| Maximum body temperature | 39.3 (38.9–39.8) | 38.9 (38.2–39.5) | .128 |
| Duration of fever (h) | 6 (12–48) | 6 (18–48) | .846 |
| Fever in ED | 20 (66.7) | 63 (45.3) | .034 |
| Final diagnosis in ED: fever without source | 6 (20.0) | 82 (59.0) | <.001 |
| Initial admission | 23 (76.7) | 91 (65.5) | .235 |
| Initial antibiotic treatment | 25 (83.3) | 57 (41.0) | <.001 |
BC, blood culture; ED, Emergency Department.
Quantitative variables are expressed as median (interquartile range); categorical variables as frequency (percentage).
Distribution and time to positivity of bacterial pathogens and contaminants isolated in the 169 patients.
| n (%) | Time to positivity (h) | |
|---|---|---|
| Bacterial pathogens (n=30) | ||
| Streptococcus pneumoniae | 9 (30.0) | 11.8 (10.2–17.9) |
| Escherichia coli | 9 (30.0) | 8.2 (4.3–11.8) |
| Streptococcus pyogenes | 4 (13.3) | 13.4 (11.4–14.5) |
| Staphylococcus aureus | 2 (6.7) | 7.6 (4.1–11.0) |
| Streptococcus agalactiae | 2 (6.7) | 11.0 (10.8–11.3) |
| Othera | 3 (10.0) | – |
| Bacterial contaminants (n=139) | ||
| Coagulase-negative Staphylococcus | 92 (66.2) | 18.0 (13.4–26.0) |
| Micrococcus spp. | 17 (12.2) | 31.0 (23.3–46.1) |
| Corynebacterium spp. | 6 (4.3) | 52.3 (36.2–61.9) |
| Streptococcus mitis | 5 (3.6) | 14.4 (9.1–19) |
| Streptococcus viridans | 4 (2.9) | 16.9 (12.4–21.5) |
| Bacillus sp | 3 (2.2) | 54.5 (32.0–62.1) |
| Pseudomonas luteola | 3 (2.2) | 11.0 (9.6–12.1) |
| Rothia dentocariosa | 2 (1.4) | 69.0 (28.8–109.2) |
| Otherb | 7 (5.0) | – |
The time to positivity is expressed as median (interquartile range).
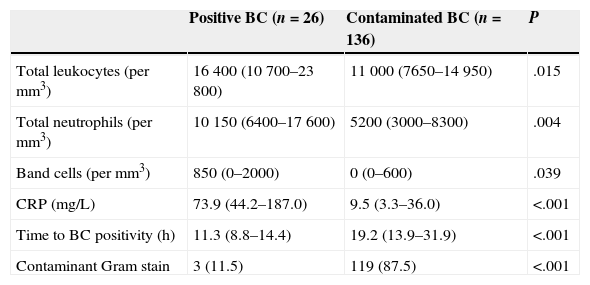
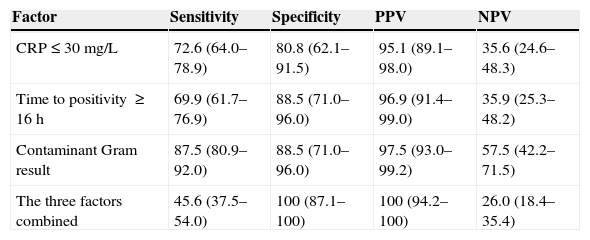
The analysis of the predictive factors for contamination excluded seven cases due to missing data: three were missing laboratory data, three had no documented time to positivity, and one had a negative Gram stain. Table 3 shows the comparative analysis of the different laboratory and microbiological variables documented in patients with a positive BC and patients with a contaminated BC. The pathogenic bacteria isolated in the three patients with Gram stain results suggestive of contamination were S. aureus (two cases) and S. pyogenes (one case); all three had been admitted for antibiotic treatment from their initial ED visit after being diagnosed with hip arthritis, scalded skin syndrome and mastoiditis, respectively. The three predictive factors for contamination that had the highest statistical significance were CRP (area under the curve [AUC], 0.81; 95% CI, 0.75–0.87; cut-off point, 30mg/L), time to positivity (AUC, 0.80; 95% CI, 0.73–0.86; cut-off point, 16h) and the initial Gram stain result. Table 4 shows the sensitivity, specificity, PPV and NPP for these three variables, individually and combined.
Laboratory and microbiological characteristics of patients with positive cultures compared to patients with contaminated cultures.
| Positive BC (n=26) | Contaminated BC (n=136) | P | |
|---|---|---|---|
| Total leukocytes (per mm3) | 16400 (10700–23800) | 11000 (7650–14950) | .015 |
| Total neutrophils (per mm3) | 10150 (6400–17600) | 5200 (3000–8300) | .004 |
| Band cells (per mm3) | 850 (0–2000) | 0 (0–600) | .039 |
| CRP (mg/L) | 73.9 (44.2–187.0) | 9.5 (3.3–36.0) | <.001 |
| Time to BC positivity (h) | 11.3 (8.8–14.4) | 19.2 (13.9–31.9) | <.001 |
| Contaminant Gram stain | 3 (11.5) | 119 (87.5) | <.001 |
BC: blood culture; CRP: C-reactive protein.
We included the 162 patients for which we had data for every parameter under analysis. Quantitative variables are expressed as median (interquartile range) and categorical variables as frequency (percentage).
Usefulness of the Gram stain result, the time to positivity of the BC and the C-reactive protein level as predictive factors for contamination of a BC with bacterial growth.
| Factor | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| CRP≤30mg/L | 72.6 (64.0–78.9) | 80.8 (62.1–91.5) | 95.1 (89.1–98.0) | 35.6 (24.6–48.3) |
| Time to positivity ≥16h | 69.9 (61.7–76.9) | 88.5 (71.0–96.0) | 96.9 (91.4–99.0) | 35.9 (25.3–48.2) |
| Contaminant Gram result | 87.5 (80.9–92.0) | 88.5 (71.0–96.0) | 97.5 (93.0–99.2) | 57.5 (42.2–71.5) |
| The three factors combined | 45.6 (37.5–54.0) | 100 (87.1–100) | 100 (94.2–100) | 26.0 (18.4–35.4) |
CRP, C-reactive protein; NPV, negative predictive value; PPV, positive predictive value.
The variables are expressed as percentage (95% confidence interval).
The subanalysis of the 33 patients at risk of OB showed that the BC was positive in three (9.1%) and contaminated in 30 (90.9%). The bacteria isolated in patients with OB were S. pneumoniae in two cases and E. coli in one. Two of the three patients with a positive BC had C-reactive protein levels above30mg/L; all three had Gram stain results suggestive of a pathogen, and the time to positivity had been documented for two patients and was less than 16h in both.
When it came to the impact of contaminated BCs on the 48 patients that had been discharged home, four (8.3%) were scheduled for reevaluation at the ED, three had the BC redone, and two were prescribed antibiotics, with one of them admitted for parenteral administration until the definitive BC results became available.
DiscussionTo the best of our knowledge, this is the first study in the literature that analyses bacteraemia in febrile children 1–36 months of age in Spain following the introduction of the 13-valent PCV. The clear predominance of contaminated over true-positive BCs in cultures with bacterial growth has been described previously by other authors after the introduction of universal vaccination with the seven-valent PCV,3,4,14,15 who reported rates of contamination even higher than the one found in our study (5:1). At the same time, the remarkable proportion of bacteraemia caused by organisms other than S. pneumoniae in our setting, in which the PCV coverage during the period of the study is estimated at 60%, has also been reported by other authors.4,7,15–18 Both circumstances underscore the importance of the early differentiation between contamination and true bacteraemia in a BC with bacterial growth.
Our study found significant differences between the contaminated BCs and the positive BCs in the time to positivity and CRP values. The times to positivity of contaminants were considerably longer than those of pathogens, with a PPV of 96.9% for a time to positivity of 16h or longer. While this finding is consistent with those of other authors in previous studies,3,9–11,19 in our study the times to positivity for pathogens and especially for contaminants were remarkably lower in comparison, as the times to positivity reported in those other studies ranged from 13.8 to 19.9h for pathogens and from 31.1 to 37.6h for contaminants. These differences can mostly be attributed to the BC monitoring systems used in the previous studies, as most of them were performed in the 1990s. In recent years, the introduction of automated continuous-monitoring BC systems has brought about a significant reduction in the time elapsed until obtaining the results accompanied by a considerable increase in the overall positive rate.20 In our study, the level of CRP was the most useful laboratory parameter for the early identification of a contaminated BC, with a PPV of 95.1% for CRP levels at or above 30mg/L. As far as we know, the use of CRP levels for this purpose has not been analysed by other authors. The studies by Sard et al. and Segal and Chamberlain3,9 showed a statistically significant difference in the total leucocyte count between contaminated and positive BCs, and the former proposed a count of less than 15000cells/mm3 as a predictive factor for contamination with a PPV of 86.8%. While we also observed a statistically significant difference in the total number of leukocytes between the two groups in our sample, we found a greater difference in CRP levels. This can be attributed to the change in the distribution of pathogens that cause bacteraemia in recent years, after which leucocyte counts have become less helpful in their identification.14,16,17 In addition to these two factors, a Gram stain result suggestive of contamination stood out as the most useful individual parameter for the early identification of a contaminated BC, with a PPV of 97.5%. This finding, consistent with what has been reported by other authors,3,10 underscores the importance of the concordance between the initial Gram stain and the final identification of the isolate.21
Compared to other studies,3,22,23 there was a considerably low percentage of patients discharged home in whom the BC was contaminated that returned for reevaluation. The reason for this is that, in the centre where the study was conducted, the microbiology department generally notifies bacterial growth in BCs only if the initial Gram stain result is suggestive of a pathogen, or if it is suggestive of a contaminant but the review of the patient's chart raises the possibility of the positive result being clinically relevant. Fluid communication between paediatrician and microbiologist is required to ensure the correct selection of patients.24
There are several limitations to this study. Firstly, it focuses on febrile patients 1–3 months of age receiving care at a tertiary paediatric hospital, so the results may not be generalisable to the paediatric population outside this age group or seen in other types of healthcare facility. Second of all, the reduced number of patients with OB precluded the validation of the predictive factors for contamination in the subgroup of patients at risk of OB. Given the drastic decline in OB in recent years, it would be necessary to conduct a prospective multicentre study to validate the usefulness of the factors proposed for this population. Lastly, the retrospective design of the study did not allow us to determine the rate of BC contamination in this sample of patients, and we could not assess factors that potentially influence the contamination of a BC, especially during sample collection and transport. However, the overall contamination rate of BCs in the ED during the period under study was below 3%,2 in compliance with the generally accepted standards.
To conclude, at present most BCs with bacterial growth in febrile patients 1–36 months of age turn out to have been contaminated. Initial Gram stain results, the time to positivity of the BC and C-reactive protein values allow the early identification of contaminated cultures, so they ought to be taken into account by paediatricians when BCs show bacterial growth. The early identification of these patients will enable a reduction in unnecessary health expenditure associated with their reevaluation.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Hernández-Bou S, Trenchs Sainz de la Maza V, Esquivel Ojeda JN, Gené Giralt A, Luaces Cubells C. Factores predictores de contaminación ante un hemocultivo con crecimiento bacteriano en Urgencias. An Pediatr (Barc). 2015;82:426–432.
Previous presentation: Partial results for this study were presented at the XVIII Reunión Anual de la Sociedad Española de Urgencias de Pediatría; April 2013; Granada, Spain.