Home-based hospital-level care, or hospital-at-home (HAH), entails a change in the classic care delivery model, promotes the rights established by the European Association for Children in Hospital Charter, prevents hospital stays and safeguards the privacy of patients and their families.1
An increasing number of hospital-level procedures are managed by HAH units, with evidence of positive results.2 The field of hospital-based internal medicine paediatrics, with its versatility and comprehensive approach to the management of hospitalised children,3 plays an essential role in the addition of new techniques and diseases to HAH.
Oncological and haematological diseases involve multiple hospitalizations that affect the quality of life of patients and their families. A study by Hansson et al.4 published in 2008 assessed the feasibility of HAH care in children with cancer in terms of patient satisfaction and preferences. Although the study did not find significant differences, the HAH group (51 patients) scored higher in fitness, psychosocial and physical health compared to the hospitalised group (44 patients).
A study conducted between 2015 and 2017 in the Alberta Children’s Hospital (Canada), included 136 children with cancer in a HAH programme to deliver chemotherapy, antibiotherapy and intravenous hydration and found favourable outcomes. Family members reported greater quality of life, less disruptions in daily routines and lower anxiety in the patient with home care.5
We conducted a retrospective review of children with haematological or oncological disease admitted to the HAH unit of a tertiary care hospital between December 2018 and February 2021. We conducted a descriptive analysis of the demographic and clinical characteristics of the sample, the treatment received and the degree of satisfaction and safety perceived by the families.
Direct home-based care was provided by paediatricians in the HAH in coordination with the oncologist in charge of the patient.
The patients in the sample met the criteria for admission to the HAH unit: clinical stability, voluntary participation in the programme and signed informed consent. The families were found to be reliable and had been trained, had telephonic access to the hospital around the clock and lived less than 30 min away from the hospital.
We obtained informed consent from families to participate in the satisfaction survey by completing an online form anonymously. The form included questions regarding satisfaction and the safety perceived by respondents, scored on a Likert scale that ranged from 1 (lowest degree) to 5 (highest degree), and the burden of care they experienced (Appendix B).
Of the total admissions to HAH (634), 7.4% (47) corresponded to patients with oncological or haematological disease; 8 required admission repeatedly, so the total patients included amounted to 31 (51% male). The median age was 8 years (IQR, 3–11), the mean stay was 7 days (IQR, 5–12). A total of 416 hospitalizations were avoided.
The most frequent oncological and blood disorders in the sample were leukaemia and neuroblastoma (76.5%). The most frequent reason for admission was the need of intravenous antibiotherapy for management of infectious diseases (70.2%) (Table 1).
Patient origin, diseases and reasons for admission to the hospital-at-home unit.
| Patient origin | |
| Oncology inpatient ward | 39 (80.8%) |
| Oncology Day Hospital | 7 (14.8%) |
| PICU | 1 (2.1%) |
| Oncological and haematological diseases | |
| Leukaemia | 25 (53.1%) |
| Neuroblastoma | 11 (23.4%) |
| Bone marrow aplasia | 3 (6.3%) |
| Bone tumour | 3 (6.3%) |
| Other (immunodeficiency. brain tumour. germ cell tumour. medulloblastoma. Langerhans cell histiocytosis) | 5 (10.6%) |
| Reason for admission | |
| Home intravenous antibiotherapy | 33 (70.2%) |
| CVC-related infection | 17 (51.5%) |
| Invasive fungal infection | 7 (21.2%) |
| Viral infection | 2 (6%) |
| Intra-abdominal infection | 2 (6%) |
| Urinary tract infection | 2 (6%) |
| Meningitis | 1 (3%) |
| Fever without source | 1 (3%) |
| Sepsis caused by Klebsiella | 1 (3%) |
| Monoclonal antibody infusion | 11 (23.4%) |
| Oxygen therapy | 1 (2.1%) |
| Intravenous fluid therapy | 1 (2.1%) |
| Nasogastric bolus feeding | 1 (2.1%) |
CVC, central venous catheter. Data expressed as absolute frequencies and percentages.
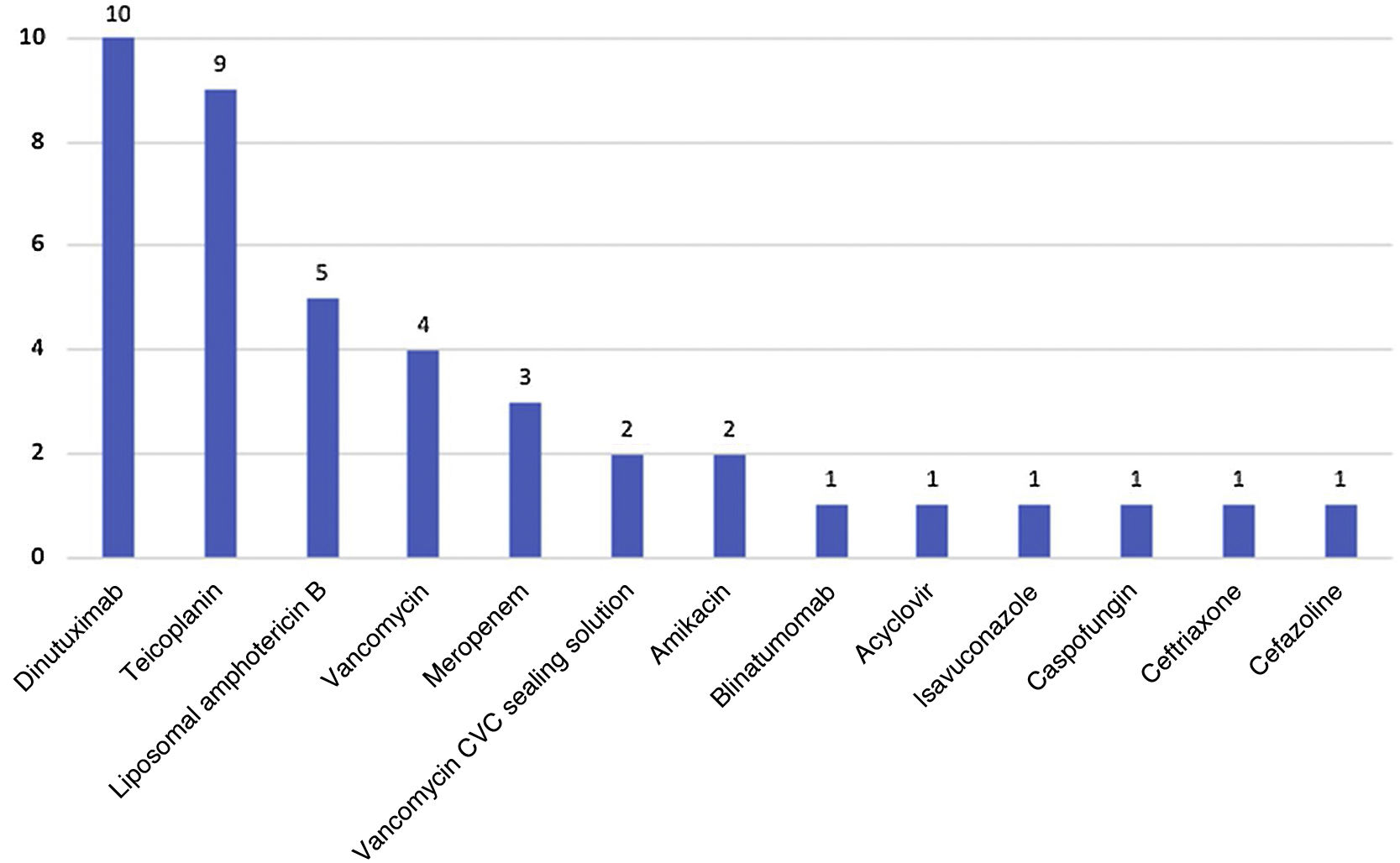
The most frequently used drugs delivered intravenously were dinutuximab and teicoplanin (Fig. 1). In 42.2% (19) of cases, these drugs were administered by the family.
During the stay in the HAH programme, 21.2% (10/47) of the patients required hospital readmission: 5 due to fever, 4 to modify treatment and 1 due to poor adherence to treatment. There were no events that compromised patient safety.
We submitted 26 invitations to complete the questionnaire, one per patient, excluding deceased patients and patients currently in relapse (3 cases), and we were unable to contact 2 of the patients. The response rate was 92% (24/26). All respondents rated their overall satisfaction with the HAH service at 4 or 5 points. Also, 52.4% expressed that the burden of care turned out to be lower than expected, while 38.1% expressed that it matched expectations. All respondents rated the perceived safety at 4 or 5 points. All would choose the option of HAH again.
As advantages, respondents highlighted an improvement in family life and caregiving balance and in comfort (19 respondents) as well as the potential avoidance of infection (2 respondents). As drawbacks, they mentioned the worry associated with not being in a hospital setting (5 respondents) and lack of confidence in managing medical devices (5 respondents), although this insecurity improved as days went by.
The main limitations of our study were the small sample size and the use of a questionnaire developed by the researchers due to the lack of a validated scale fitting the needs of the study.
We conclude that paediatric patients with oncological disease may benefit from admission to HAH units, which can allow a reduction in hospital stays with a high level of satisfaction and perceived safety in the families.
Further studies are required to establish the effectiveness and safety of HAH in this group of patients.