Vaccination has marked a tipping point in the SARS-COV-2 pandemic, as it turned out to be highly effective in the prevention of severe forms of coronavirus disease 2019 (COVID-19), chiefly evinced in a reduction in the hospitalization rate and mortality associated with the infection, but also through a certain impact on its transmission. Given the limited number of doses available, the global COVID-19 vaccination strategy prioritised the most vulnerable groups and essential workers, thereby excluding healthy children, whose risk of severe disease is substantially lesser than the risk in adults.1 This is not to say that children have not been affected by COVID-19, and we must not forget the dramatic unexpected consequences of COVID-19 in the paediatric population, a serious “silent pandemic” in which basic rights as are education or socialization have been violated and in which an absolute focus on COVID has relegated children to the background, in some instances failing to remember that they continue to fall ill and suffer all the other diseases that existed before, which could exacerbate the universal decrease in the vaccine coverage for vaccines included in the routine immunization programme.2
In today’s world, and especially in high-income developed countries, the recommendation for routine vaccination of children and adolescents against SARS-COV-2 appears to be a process almost inextricably tied to the inclusion of this indication in the summary of product characteristics of the vaccines that are currently available. As of December 2021, 2 messenger RNA (mRNA) vaccines against SARS-COV-2 have already been authorised in Europe for administration starting at age 12 years (Pfizer’s BNT162b2 and Moderna’s mRNA 1273) and 1 for administration from age 5 years (Pfizer’s BioNTech).1 Other vaccines are also used in children outside Europe, such as the inactivated vaccines in China (Sinovac-CoronaVac and BBIBP-CorV) already used from age 3 years, or Covaxin (inactivated vaccine with an adjuvant) and ZycovD (DNA vaccine) in India, authorised for use from age 12 years. These and other vaccines are still in trials for administration in children as young as 6 months.1 Having the necessary data on the use of these vaccines in the paediatric population is a non-negotiable requirement for, when the time comes and if indicated, they can be used in children and adolescents with the same guarantees offered to the adult population, but it does not entail that recommendations will immediately be issued for their use in clinical practice, recommendations that, on the other hand, need not be linked to the age limits established by the sponsor, which are usually arbitrary and not necessarily relevant in the pathophysiology of COVID-19 in particular.
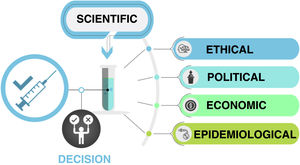
The decision whether to routinely vaccinate all children against COVID-19 is complex and depends on scientific, ethical, political, epidemiological and economic factors (Fig. 1 and Table 1). The infection is asymptomatic or mild in most paediatric cases, and fewer than 2% of symptomatic paediatric patients require hospital admission, many of which happen to have COVID-19 but are admitted for other reasons.3 Although the infection, like any other, is not absolutely without risk, most severe cases of COVID-19 in the paediatric population occur in children with other risk factors and comorbidities, as is the case in adults.3 Therefore, the vaccination against COVID-19 of children with risk factors or comorbidities is not up for debate, as their risk status places them in the same level of priority as other risk groups, independently of age.1 As soon as the vaccines are approved for use in the paediatric age group, an active search should be performed to identify children at risk and offer them vaccination. Children with cancer, immunosuppression, obesity, Down syndrome or severe neurologic disease are some of the established priority groups, but not all high-risk conditions have been identified in children as clearly as in adults.3 For this reason, in any case in which there is uncertainty about the specific condition and the associated risk, the most prudent and advisable course of action after an individualised evaluation of its indication is to vaccinate. Similarly, it would be reasonable to consider children and adolescents that live with individuals who are immunosuppressed or at high-risk of severe COVID-19 a high-priority group for vaccination.
Factors influencing the decision to universally vaccinate children against COVID-19. In addition to the scientific evidence demonstrating the safety and effectiveness of vaccination in the paediatric age group, there are other factors at play, including epidemiologic factors (chiefly the prevalence of disease, the impact on the health care system and global vaccine coverage), economic factors (accessibility of vaccines and associated costs), ethical factors (equity) and political factors. (Original image created by Dr Alberto Gómez Carballa).
Summary of the arguments in support and against universal vaccination against SARS-COV-2 in the paediatric population.
| Criteria | In support | Against |
|---|---|---|
| Burden of disease | Although the global burden of disease in children is low, it is not negligible. | The direct benefits of vaccination are very limited and most severe cases occur in children with comorbidities and/or risk factors |
| Multisystem inflammatory syndrome in children temporally associated with SARS-COV-2, while rare, is severe. | It is not known yet whether vaccination can prevent multisystem inflammatory syndrome in children temporally associated with SARS-COV-2 | |
| Practical | Promote unrestricted lifestyles in children, reducing the duration of quarantine periods, allowing free movement, lowering the intensity of other measures. | These practical aspects could be pursued with other measures, taking into account the particular characteristics of children |
| Equity - ethical | Choosing not to vaccinate children, when vaccination is an option, does not guarantee equity | Vaccinating children contributes to inequity, as there are other groups with higher priority and the global supply of vaccines is limited |
| Lowering the minimum requirements for inclusion in the routine childhood immunization schedule should favour the inclusion of other vaccines that are more important | The COVID-19 vaccination follows an independent financing line from the other vaccines, but may further postpone the inclusion of other more priority vaccines in children. | |
| Transmission | Potential impact on disease transmission and contribution to indirect protection | Few objective data support the impact on transmission, especially in children under 9−10 years |
| Post-COVID and/or other sequelae | Potential benefits of vaccination in the prevention of paediatric long COVID | There is no clear evidence on the prevalence of long COVID in children, that its severity is comparable to adult long COVID or that it could be prevented by the vaccine |
| It is possible that there are additional sequelae that have yet to be identified | New sequelae have not been identified and large studies in the paediatric population have already been published | |
| Economic | Accelerate the return to pre-pandemic activity and stability | Prioritizing resources for vaccination against SARS-COV-2, delaying introduction of other vaccines |
| Safety | The vaccines are safe. The cases of myopericarditis observed in vaccinated individuals aged more than 12 years have been self-limited and benign, and this event has not been observed to date in children aged 5–12 years | Myopericarditis associated with vaccination in the paediatric population, although rare, has been observed in vaccinated children over 12 years and may have a negative impact in terms of vaccine trust in the population for this and other vaccines |
| Proactive action before emergence of new variants | Should new variants emerge that were particularly harmful to children, it would be beneficial for children to have been vaccinated beforehand | None of the variants that have emerged to date has exhibited a different behaviour in children, and none should be necessarily expected |
| Endemicity | In the context of endemic disease, leaving children unvaccinated provides a potential reservoir for the emergence of new variants | Given that disease is mild in children, leaving them as a potential reservoir would result in mild primary infection and a natural booster of immunity in adults |
On the other hand, the direct benefits of universal vaccination of healthy children are marginal compared to universal vaccination of adults.1,3,4 The burden of disease is low and mild, with the exception of multisystem inflammatory syndrome in children temporally associated with SARS-COV-2 which, while rare, is associated with a high mortality (1%–2%) and develops in paediatric patients without identifiable risk factors, and it is not currently known how effective vaccines may be at preventing this form of disease.3 Furthermore, children do not seem to be a major reservoir of the infection, and the observed pattern of transmission is from adults to children, and not the other way around.1 The actual contribution of children to the herd immunity against SARS-COV-2 eagerly pursued through vaccination remains unknown, although in the case of adolescents, it should not be any lesser than that of young adults. However, utmost caution should be used in making the decision to vaccinate all healthy children against COVID-19, especially in a context like the one found in Spain, where the vaccine coverage in the target population is very high and the global incidence of disease is low, which further limits the marginal benefits of this strategy in comparison to the potential adverse effects of vaccines and the impact on vaccine trust in the population. Alternatively, if vaccination does not extend to children, 25% of the global population will not be vaccinated, which could give rise to a specific reservoir for the virus to evolve, which may or may not be a problem or a potential solution in the context of endemic SARS-COV-2 infection, by restricting infection to an age group where its impact is mild and providing natural boosters to immunity in adults.5
There are also significant ethical considerations when it comes to the implementation of universal vaccination of the paediatric age group when vaccine coverage in the poorest countries has yet to reach 10%.1 We will only succeed in containing the pandemic if we act globally and equitably, and vaccination of healthy children seems unacceptable as long as there is a single child or adult at high risk in the world that has not yet received a first dose of vaccine. In addition, not taking this approach all but ensures that SARS-COV-2 can keep evolving and that new variants will emerge and threaten what has been achieved in regions that have advanced further in controlling the pandemic. Nevertheless, the fact that vaccination against SARS-COV-2 in healthy children is not a priority compared to vaccination of other groups or administration of other vaccines does not mean that it would not be beneficial for children or that refraining from vaccinating them once the health authorities allow it would resolve the current situation of global inequity.
The immunization guidelines of the Advisory Committee on Vaccines of the Asociación Española de Pediatría (Spanish Association of Pediatrics) are the recommendations followed by most paediatricians and families in Spain, and currently include vaccination against SARS-COV-2 in children and adolescents.6 It should therefore be implemented, if it is officially recommended by the Spanish health authorities, but this should not detract from the efforts or the priority that should be given to other vaccines that are more important for our children. For, when our children and their protection is concerned, the health authorities have the opportunity to update immunization schedules and include other vaccines whose benefits are no longer debatable: the rotavirus vaccine for all infants, the meningococcal B and ACWY vaccines for infants and adolescents, the HPV vaccine for boys, and not only girls, or the flu vaccine, among others. We must make every effort to keep vaccine coverage rates from declining, but also to ensure that the official routine childhood immunization schedule in Spain be as complete as possible, and comparable to the offering in neighbouring countries. Now, more than ever, in the “COVID-centric” world we are currently living in, children need for us paediatricians to be their advocates and guarantors in health.
Conflicts of interestFMT has received fees from Biofabri, GSK, Pfizer Inc, Sanofi Pasteur, MSD, Seqirus, Novavax and Janssen as an advisor, consultant or presenter unrelated to the objectives of the present work. He has also been principal investigator in clinical trials endorsed by the pharmaceutical companies mentioned above in addition to Ablynx, Regeneron, Roche, Abbott and MedImmune, with all fees paid directly to the institution where the work was conducted.
Please cite this article as: Martinón-Torres F. Vacunación pediátrica frente al COVID-19 y a pesar del COVID-19. An Pediatr (Barc). 2022;96:4–7.