The influence of parental obesity on their obese offsprings is acknowledged but insufficiently characterised.
Patients and methodsRetrospective study of 800 obese patients (45.2% girls; age: 10.35±3.40 years, body mass index [BMI]: +4.22±1.68 standard deviation score [SDS]). Group comparison according to the presence of obesity in none (n=347) or any of the parents (n=453), and then whether the obese parent was the father (n=185), the mother (n=151), or both parents (n=117) were performed. The parameters analysed were: Age at the onset of the obesity and at their first visit, birth weight (BW), BMI-SDS, blood glucose, insulin level, homeostatic model assessment (HOMA) index, total cholesterol (TC), HDL, LDL, triglycerides, 25-OH-vitamin-D, area under the curve (AUC) for insulin in the oral glucose tolerance test (OGTT), whole body insulin sensitivity index (WBISI), LDL/HDL and TC/HDL ratios, and weight loss after 12 month follow-up.
ResultsNo differences were observed between groups as regarding gender, ethnic background, or pubertal stage. Patients with one obese parent showed higher BW-SDS and BMI-SDS (P<.01), more severe impairment of carbohydrate metabolism (blood insulin, insulin-AUC, HOMA, HbA1c [P<.01] and lower WBISI [P<.05]) than those with no obese parent. Among those patients with a single obese parent, higher BW-SDS, insulin, HOMA, and lower 25-OH-vitamin D (P<.05) was observed when obesity was present in the mother. There was a higher prevalence of metabolic syndrome when both parents were obese (χ2=5.96, P<.05). A total of 132 patients reduced their BMI by ≥1.5 SDS, or their weight by ≥10%, with no influence of the background of parental obesity.
ConclusionsObesity in any parent determines a higher severity of their offspring obesity and metabolic comorbidities, more importantly when obesity is present in the mother or in both parents, but without interference in the options of therapeutic success.
La influencia de la obesidad parental sobre la de los hijos y sus comorbilidades, aunque asumida, está insuficientemente caracterizada.
Pacientes y métodosEstudio retrospectivo de 800 pacientes obesos (45,2% niñas; edad: 10,35±3,40 años, índice de masa corporal [IMC]:+4,22±1,68 standard deviation score[SDS]). Se realizaron comparaciones entre grupos según la presencia de obesidad en ningún (n=347) o algún progenitor (n=453), diferenciando entre presencia de obesidad en el padre (n=185), la madre (n=151) o ambos progenitores (n=117). Variables consideradas: edad al inicio de la obesidad y en primera consulta, peso neonatal (PRN), IMC-SDS, glucemia, insulinemia, índice homeostatic model assessment (HOMA), colesterol total (CT), HDL, LDL, triglicéridos, 25-OH-vitaminaD, área bajo la curva (AUC) de insulinemia en el test de tolerancia oral a la glucosa (TTOG), whole body insulin sensitivity index (WBISI), cocientes LDL/HDL y CT/HDL y reducción ponderal en los 12 primeros meses de seguimiento.
ResultadosNo hubo diferencias en la distribución por sexo, etnia y pubertad entre grupos. Aquellos pacientes con algún progenitor obeso presentaron mayor PRN-SDS e IMC-SDS (p<0,01), mayor afectación del metabolismo hidrocarbonado (insulinemia, AUC-insulina, HOMA, HbA1c [p<0,01] y menor WBISI [p<0,05]) que aquellos sin ningún progenitor obeso. Entre aquellos con un único progenitor obeso, se observó mayor PRN-SDS, insulinemia y HOMA y menor 25-OH-vitaminaD (p<0,05) cuando el antecedente era materno. Existía mayor prevalencia de síndrome metabólico cuando ambos progenitores eran obesos (χ2=5,96, p<0,05). De todos ellos, 132 disminuyeron el IMC≥1,5SDS y/o el peso ≥10%, sin influencia del antecedente de obesidad parental.
ConclusionesLa obesidad en algún progenitor determina mayor gravedad de la obesidad y de las alteraciones del metabolismo hidrocarbonado en sus hijos; acentuándose cuando la obesidad es materna o de ambos progenitores, pero sin influir en la posibilidad de éxito terapéutico.
The socioeconomic changes that have taken place in recent decades have led to the development of new dietary habits and more sedentary lifestyles, which in turn facilitate the development of obesity in current generations of children and adolescents.1 In addition, children of obese parents may be at an even higher risk of obesity due to the combination of the inluence of dietary habits in the household and a greater genetic susceptibility to the disease. In fact, a considerable percentage of the children and adolescents that are currently obese have obese parents.
It has been well established that certain dietary and nutritional patterns in families can trigger obesity in their younger members. Methods involving direct observation have been used to identify behavioural components in family meals (interpersonal relationships and relationships with food) that are beneficial or deleterious as concerns childhood obesity,2 evidencing that the influence of the family extends beyond the parents. Children who live with grandparents, figures who are increasingly involved in their care, are at greater risk of developing obesity.3 Grandparents tend to indulge and overfeed their grandchildren and to spare them from physical tasks, increasing their risk of obesity. The underlying motivation for this behaviour is their affection for the child and is influenced by personal experiences, misunderstandings and a low awareness of the adverse health effects of obesity.3
Parents and their weight status influence the development of obesity in their children as well as the outcome of treatment. Various studies have highlighted differences between parents in their perception of paediatric obesity as a disease and in their attitude towards obesity in their children, but did not assess the presence of obesity in the parents themselves. Broadly speaking, a large percentage of parents have an inaccurate perception of the weight status of their children, which is associated with a 12-fold increase in the probability of having an obese child,4 and delegate the responsibilities associated with paediatric obesity to schools and public institutions.5 Recent studies in children and adolescents enrolled in weight loss programmes with parental involvement have reported that non-obese parents have a better understanding of the interventions involved in the treatment of obesity associated with better communication with the provider, including more careful listening and spending the necessary time to explain the recommendations on the part of the latter.6 There are also studies demonstrating that weight loss programmes are less likely to be successful in children with obese parents or siblings, and some have described a poorer response to treatment in association with maternal obesity. Parental comorbidities correlated to obesity have also been associated with poorer outcomes of treatment of paediatric obesity, with a stronger association when both parents had comorbidities. Other studies have established that parental weight loss has a significant impact on treatment outcomes in obese children. Overall, a small number of studies suggest that parental obesity may influence the outcomes of treatment of obesity in children and adolescents. However, the role of parental obesity in the severity of paediatric obesity and its associated comorbidities is still under debate, and its potential impact on treatment outcomes has yet to be adequately established.7
For this reason, the main goal of our study was to analyse the severity of obesity, the presence of metabolic comorbidities and the response to treatment in obese children and adolescents in relation to the presence of obesity in one or both parents, additionally assessing the potential influence of the specific parent affected by obesity.
Patients and methodsWe conducted a retrospective study in 800 obese patients (361 [45.2%] female and 439 [54.8%] male) aged 10.35±3.40 years (range, 0.33–17.84 years) and with a body mass index (BMI) z-score of 4.22±1.68 managed in the obesity clinic of the Department of Endocrinology in a tertiary care hospital between 2009 and 2014, recording the BMI of parents after measuring the weight and height of both parents, and with a 1-year follow-up. Of all the included patients, 434 (54.2%) were in the prepubertal stage (Tanner stage I) and 366 (45.8%) in the pubertal stage (Tanner stage≥II). Most of the patients in the cohort were Caucasian (n=599; 72.7%) or Hispanic (n=179; 21.7%).
In every patient, the following variables were recorded and standardised in the initial visit to the clinic: weight, height, BMI8,9 and systolic and diastolic blood pressures10 (mean of 3 measurements taken with a Critikon®-Dinamap™ 8100 vital signs monitor). We considered patients obese if their BMI z-score was 2 or greater based on Spanish reference growth Charts8 and the standards of the International Obesity Task Force (IOTF).9 We retrieved the age at onset of obesity and the birth weight (BW), which we standardised for sex and gestational age.11 The initial evaluation included measurement of the serum levels of glucose, insulin, glycated haemoglobin (HbA1c), uric acid, total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, triglycerides, 25-hydroxy vitamin D, thyroid-stimulating hormone (TSH) and free thyroxine (fT4) after 12h fasting. AWe did not find significant differences between the groups(OGTT; 1.75mg glucose/kg weight to a maximum of 75g). We calculated the area under the curve (AUC) of glucose and insulin applying the following formula: 0.25×basal level+0.5×half-hour value+0.75×1-hour value+0.5 2-hour value. We calculated the Homeostatic Model Assessment (HOMA) index as basal glucose (mg/dL)×basal insulin (μIU/mL)/405, and the whole-body insulin sensitivity index (WBISI) as 10000/(fasting glucose×fasting insulin×mean OGTT glucose×mean OGTT insulin)1/2.12 Metabolic syndrome (MS) was diagnosed in patients aged more than 10 years (n=454; 56.8%) according to the criteria proposed by the International Diabetes Federation (IDF).13
Once obesity was diagnosed and underlying organic diseases excluded, all patients were managed with conservative treatment based on 3 main components: (1) behavioural modification with the aim of eating slower, eating smaller portions and organising the timing of meals (based on classical conditioning, reinforcement theory and the identification and control of negative automatic thoughts); (2) dietary recommendations (adequate distribution of food groups in a weekly programme without specific calorie restrictions, with energy restriction pursued indirectly through slower eating), and (3) physical indication (prescription of 1h a day of aerobic play or exercise adapted to the age and circumstances of the patient, recommending a progressive increase in intensity). Follow-up clinical evaluations were scheduled as follows, independently of the severity of obesity: initial visit, first follow-up evaluation at 1 month, and follow-up evaluations every three months thereafter. We encouraged all members of the family to get involved in implementing the given recommendations with the aim of facilitating adherence in the patient.
We considered that patients had achieved significant weight loss if they succeeded in decreasing their BMI z-score by 1.5 points and/or their weight by 10% or more in the 12 months of follow-up.
We compared patients based on parental obesity (BMI>30kg/m2)14 initially classifying them in 2 groups (no parent with obesity [NO group, n=347] versus at least 1 parent with obesity [OB group, n=453]), subsequently comparing patients in the OB group based on whether obesity was present in only 1 parent (1-OB; n=336) or in both (2-OB; n=117). In the subset with only 1 obese parent, we compared patients with an obese father (F, n=185) to patients with an obese mother (M; n=151).
We performed the statistical analysis with the Statistical Package for Social Sciences (SPSS) version 15.0 (MapInfo Corporation, Troy, NY, USA). We used the Kolmogorov–Smirnov test to assess the normality of the distribution of the variables under study (the variables fitting a normal distribution were blood glucose, TC and LDL cholesterol). For normally-distributed variables, we compared independent groups by means of the Student t test, and for all others (with a non-normal distribution) we used the Mann–Whitney U test. We compared the proportions of patients with MS in different groups using the χ2 test. We defined statistical significance as a P-value of less than .05.
Although the study was to include every patient meeting the specified criteria managed in the 2009–2014 period, we still estimated the sample size required to achieve the established objectives. Assuming a prevalence of hyperinsulinaemia of 30% in the group of patients without obese parents, the size required per group to detect an expected increase of 10% in the prevalence of hyperinsulinaemia in patients with at least 1 obese parent versus those without obese parents with an alfa error of 5% and a beta error of 10% was n=362.
ResultsWe did not find significant differences between the groups (NO vs OB; 1-OB vs 2-OB; F vs M) in sex, ethnicity and pubertal development distributions or in the ages at onset of obesity and at the initial visit (Table 1).
Comparison of patients by parental obesity group.
| 2 non-obese parents (n=347) | At least 1 obese parent (n=453) | 2 obese parents (n=117) | Obese father (n=185) | Obese mother (n=151) | |
|---|---|---|---|---|---|
| Age (years) | 10.27±3.5 | 10.37±3.31 | 10.06±3.61 | 10.39±3.27 | 10.59±3.11 |
| Age at onset of obesity (years) | 6.41±3.2 | 6.25±3.11 | 6.28±3.01 | 5.99±3.33 | 6.55±2.9 |
| BW z | 0.14±1.4 | 0.39±1.45 | 0.7±1.6 | 0.13±1.26 | 0.45±1.5 |
| Height z | 0.94±1.28 | 1.04±1.18 | 0.97±1.28 | 1.09±1.09 | 1.03±1.23 |
| Target height z | −0.26±0.97 | −0.34±0.98 | −0.23±0.96 | −0.23±0.96 | −0.40±1.00 |
| BMI z | 3.91±1.46 | 4.46±1.80 | 4.82±2.07 | 4.20±1.52 | 4.52±1.87 |
| Basal glucose (mg/dL) | 92.60±6.53 | 92.89±7.04 | 92.02±7.17 | 92.87±6.48 | 93.59±7.55 |
| Basal insulin (μU/mL) | 13.05±6.80 | 15.45±11.15 | 16.52±12.76 | 14.02±7.6 | 16.31±13.10 |
| HOMA | 3.01±1.64 | 3.59±2.75 | 3.80±3.00 | 3.23±1.87 | 3.84±3.35 |
| Insulin AUC (μU/mL) | 84.50±49.33 | 101.49±64.22 | 103.47±61.85 | 93.52±56.54 | 109.17±73.23 |
| WBISI | 3.79±1.99 | 3.41±1.75 | 3.18±1.65 | 3.64±1.79 | 3.31±1.78 |
| Total cholesterol (mg/dL) | 158.44±28.68 | 159.04±31.01 | 158.98±32.75 | 161.29±31.02 | 156.26±29.54 |
| LDL cholesterol (mg/dL) | 96.34±24.29 | 96.76±25.97 | 98.09±28.60 | 97.74±24.36 | 94.49±25.71 |
| Triglycerides (mg/dL) | 77.97±50.52 | 82.94±52.02 | 82.53±48.46 | 83.37±56.10 | 82.72±49.76 |
| 25-hydroxy vitamin D (ng/mL) | 23.05±9.45 | 21.15±8.31 | 23.37±10.37 | 22.89±7.96 | 19.40±8.35 |
AUC, area under the curve; BMI, body mass index; BW, birth weight; WBISI, whole body insulin sensitivity index; z, z-score.
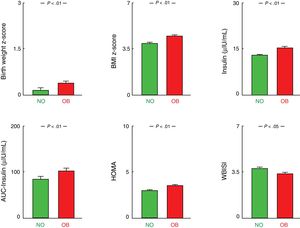
Patients in the OB group, that is, with at least one obese parent (father, mother or both) had higher BW z-scores and higher BMI z-scores at the time of the first visit compared to those without an obese parent (NO vs OB, P<.01). We also found a higher frequency of carbohydrate metabolism abnormalities in the OB group, with higher values of blood insulin, the insulin AUC, the HOMA index and HbA1c (all with P<.01) and lower values of the WBISI (P<0.05), although the blood glucose levels and the glucose AUC were similar in both groups (Table 1; Fig. 1). Triglyceride levels tended to be higher in patients in the OB group, but the difference was not statistically significant (P=.076). We did not find any differences in any of the other variables under study. When we compared patients without obese parents with patients whose parents were both obese (NO vs 2-OB), we found similar results (Table 1).
Number of obese parentsWe found a higher prevalence of MS (χ2=5.96; P<.05) in patients with 2 obese parents (23.08%) compared to patients with one obese parent (15.77%) and patients without obese parents (13.54%). Patients with 2 obese parents had higher BW z-scores (P<.01) and higher BMI z-scores in the initial visit (P<.05) compared to patients with a single obese parent. When it came to metabolic comorbidities, patients with 2 obese parents had higher TC/HDL ratios (P<.05) (Table 1; Fig. 2). We did not find any significant differences in any of the other variables under study (Table 1).
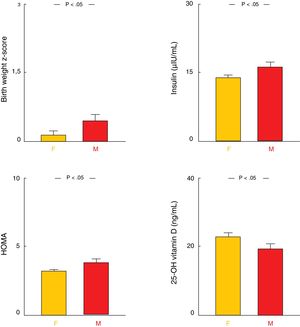
Influence of the affected parentIn the group of patients with 1 obese parent, we found higher BW z-scores, insulin levels, and HOMA index values and lower levels of 25-hydroxy vitamin D in the subset in whom the obese parent was the mother (P<.05 for all analyses). Patients with an obese mother also tended to have higher BMI z-scores, but this difference was not significant (Table 1; Fig. 3). We did not find statistically significant differences in any of the other variables under study.
Response to treatmentOf the total patients in the cohort (n=800), 132 (16.5%) achieved a significant weight loss in 12 months of treatment (reduction of BMI z≥1.5 points and/or body weight≥10%), and we found no significant differences in the percentage of patients that achieved significant weight loss based on the number of parents with obesity (0, 1 or 2).
DiscussionThe results of our study show that parental BMI has an impact on some characteristics of paediatric obesity and its comorbidities.
Children and adolescents with at least one obese parent had higher BWs and more severe obesity (based on the BMI) at the time of the initial consultation. These 2 features were exacerbated in patients with 2 obese parents. Although this association is controversial, it is supported by the work of other authors, especially as concerns the impact on younger children (age<10 years), and it may also be important in the development or persistence of obesity in adulthood.15–17
In the group with a single obese parent, when the affected parent was the mother, children and adolescents had higher BWs and tended to have a higher BMI at the time of seeking care for obesity. The association of maternal obesity with foetal macrosomia and an increased risk of obesity and metabolic comorbidities later in life is well established.18,19 Our study suggests that this association is stronger when the mother is affected as opposed to the father. This maternal influence may be explained—at least in part—by the presence of prenatal and perinatal factors associated to the development of obesity in future stages of life.20–22
As for the disorders of carbohydrate metabolism associated with obesity, insulin resistance (IR) was more severe in patients in the OB group. However, this was not the case of blood glucose levels, which were similar across de sample and independent of parental obesity. Despite the challenges in defining IR described by some authors,23 previous studies conducted by our group24,25 and collaborative studies conducted in Spain26 have demonstrated that IR is much more frequent than abnormal glucose levels in obese children (a feature that differentiates this population from adults). Furthermore, this outcome corroborates the findings of previous studies that have also described an increased risk of IR and a higher prevalence of hyperinsulinaemia in patients with obese parents, associating this factor with other cardiovascular risk factors (obesity, dyslipidaemia and high blood pressure) characteristic of MS, which in our cohort was more prevalent in patients whose parents were both obese,27–29 although it is important to consider that Halvorsen et al. also found an association between the presence of cardiovascular risk factors and MS in parents and children, independently of the BMI of either. In the opinion of Monzani et al., parental obesity is the sole risk factor required for the development of MS in childhood, unlike adolescence, when obesogenic habits are also at play.
We ought to mention that the definition of MS used in our study was restricted to patients aged more than 10 years, and that the prevalence found would have been higher had we applied paediatric criteria covering a broader age range. We chose to apply the criteria of the IDF to define MS due to the current lack of consensus in the international literature regarding the appropriateness of diagnosing MS in the paediatric age group. Thus, while specific scientific criteria have been proposed for its definition in the paediatric population, there are also authors that disagree with the use of this diagnosis at early ages and instead recommend (as does the IDF) the diagnosis and treatment of each individual metabolic disorder, refraining from the use of MS as a diagnostic category.13
In our sample, parental obesity was also associated with a higher prevalence of MS, an association that was strongest when both parents were obese, thus representing an increased risk of future cardiovascular and metabolic disease in children and adolescents. In fact, the prevalence of MS in our cohort was higher compared to other studies that have also found an association between MS and obesity in one or both parents.30,31 This could be related to the greater severity of obesity in the patients included in our study (as evinced by the high mean BMI z-score) and to our sample coming from a hospital cohort where the detection of metabolic disturbances by the primary care clinicians that refer patients to specialty care may have been a source of bias. In spite of this, we found no significant differences between groups in the lipid profile, save for a higher TC/HDL ratio in patients with two obese parents, contrary to other studies that did find higher triglyceride and LDL-cholesterol levels and lower levels of HDL-cholesterol in children and adolescents with obese parents and with MS,27,28 although this could have been due to the lower mean age of the patients in our study.
The comparison of metabolic comorbidities in patients with one obese parent showed that IR was more severe in cases associated with maternal obesity. This finding corroborates previous evidence demonstrating the influence of maternal obesity on the development of IR and obesity, even in children born with a normal weight for gestational age,32 and contributes novel information on the difference in comparison with paternal obesity.
Another salient finding was that the levels of 25-hydroxy vitamin D were lower in patients whose mothers were obese. The association between obesity and vitamin D is a subject that currently attracts considerable interest, including obesity in the collection of risk factors for vitamin D insufficiency and deficiency.33 These risk factors may affect several members of a family sharing similar dietary and leisure habits. However, the association between maternal obesity and the levels of 25-hydroxy vitamin D in children has not been studied extensively, although there is evidence that the presence of vitamin D deficiency in the mother secondary to obesity during pregnancy may play a role in this association, among other possible factors.34
Contrary to expectations, a novel finding of our study was that a positive parental history of obesity had no impact on the likelihood of responding favourably to conservative treatment, as previously described, in the 12 months of follow-up. This finding diverged from the scarce data on the subject reported in the literature, which suggests that parental obesity is associated with poorer outcomes of treatment in obese children and adolescents.7 There is no question that the involvement of the family, independently of the BMI, is one of the key factors that determine a satisfactory response to treatment.35–37 However, our results suggest that parents with obesity are also capable of promoting healthy habits in their offspring, with a success rate similar to that found in non-obese parents. Furthermore, witnessing the challenges and the comorbidities experienced by their parents may inspire children (especially older ones) to take measures for weight control independently. However, keeping records of the degree to which the parents of patients implemented the recommendations and of changes in the weight status of the former would have provided additional information on this aspect, and the lack of such records was a limitation of our study.
Our study is not free from the limitations intrinsic to cohort studies, including the impossibility of establishing causal relationships (only association can be established) and the time required to achieve the necessary sample size and complete the follow-up, which may have an impact on the homogeneity of the conditions under which subjects participate in the study.
In conclusion, the results of our retrospective study on a large cohort of paediatric patients with obesity demonstrate that a history of obesity in a parent is associated with greater severity of paediatric obesity and the associated IR in their obese offspring, an association that is stronger when it is the mother or both parents who are obese. Nevertheless, a positive parental history of obesity does not have a negative impact on the probability of successful treatment.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martínez-Villanueva J, González-Leal R, Argente J, Martos-Moreno GÁ. La obesidad parental se asocia con la gravedad de la obesidad infantil y de sus comorbilidades. An Pediatr (Barc). 2019;90:224–231.
Previous presentation: This study was presented at the 37th Congress of the Sociedad Española de Endocrinología Pediátrica (SEEP), Valencia, Spain, 2015.