The presence of a haemodynamically significant patent ductus arteriosus (hsPDA) in preterm newborns (PTNBs) is associated with prolonged need of mechanical ventilation, bronchopulmonary dysplasia (BPD), necrotising enterocolitis (NEC), metabolic acidosis, intraventricular haemorrhage (IVH), pulmonary haemorrhage and periventricular leukomalacia.1 For this reason, pharmacological closure of the ductus is common practice, usually through administration of non-selective cyclooxygenase (COX) inhibitors, indomethacin or ibuprofen. Since these drugs may cause adverse events, other agents, such as paracetamol, are being investigated that may be safer while still effective. In this article, we describe our experience with paracetamol in these patients.
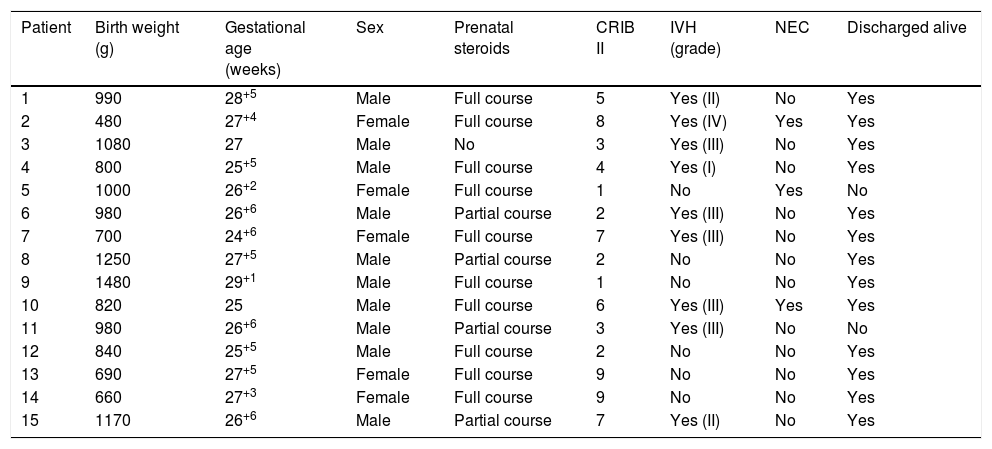
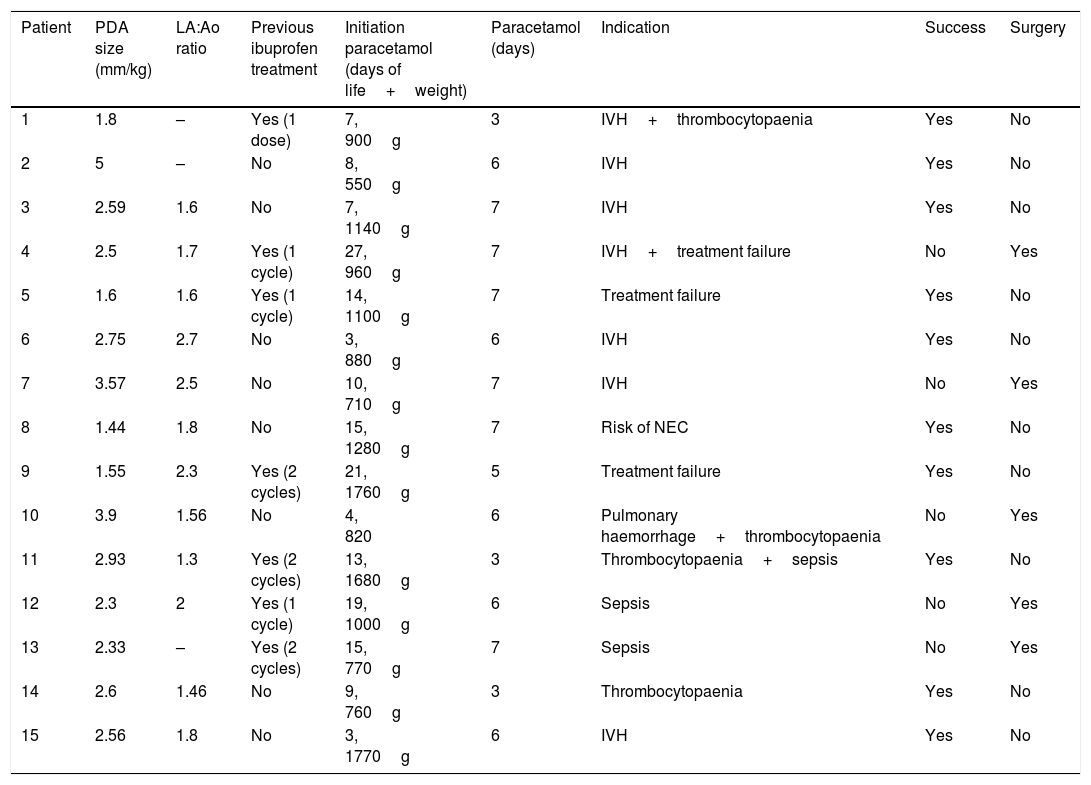
In our unit, active measures for closure of PAD are implemented in PTNBs that develop symptoms or with sonographic signs of moderate to severe haemodynamic compromise in whom spontaneous closure is unlikely. The first line of treatment is ibuprofen, which is delivered intravenously. In the last 4 years, we have used paracetamol by the oral or intravenous route in 15 PTNBs with satisfactory results. In all these newborns, paracetamol was administered because treatment with ibuprofen had failed or the patient had contraindications for it. Parents gave consent to the use of paracetamol after being informed that it was an off-label use of the drug. Table 1 summarises the basic characteristics of the patients. The mean gestational age at birth was 26+4 weeks (median, 26+6 weeks; range 24+6–29+1 weeks) and the mean birth weight was 928g (median, 980g; range, 480–1480g). The most frequent indication for treatment with paracetamol (7/15 patients) was a recent history of IVH, which had been severe in 6/15 patients. Three patients developed NEC and 2 died. Table 2 summarises the findings of the sonographic assessment of PDA and the main treatment-related variables. Half of the patients had been previously treated with ibuprofen. Paracetamol was given at a dose of 15mg per kilogram of body weight every 6h. We considered that closure of the PDA was successful if complete closure was achieved, or if the hsPDA improved to a minor PDA with no haemodynamic effects and requiring no further treatment. In our case series, successful closure was achieved in 10/15 patients, with the remaining 5 requiring surgical closure. None of the patients had side effects that could be attributed to paracetamol in the short term.
Patient characteristics.
| Patient | Birth weight (g) | Gestational age (weeks) | Sex | Prenatal steroids | CRIB II | IVH (grade) | NEC | Discharged alive |
|---|---|---|---|---|---|---|---|---|
| 1 | 990 | 28+5 | Male | Full course | 5 | Yes (II) | No | Yes |
| 2 | 480 | 27+4 | Female | Full course | 8 | Yes (IV) | Yes | Yes |
| 3 | 1080 | 27 | Male | No | 3 | Yes (III) | No | Yes |
| 4 | 800 | 25+5 | Male | Full course | 4 | Yes (I) | No | Yes |
| 5 | 1000 | 26+2 | Female | Full course | 1 | No | Yes | No |
| 6 | 980 | 26+6 | Male | Partial course | 2 | Yes (III) | No | Yes |
| 7 | 700 | 24+6 | Female | Full course | 7 | Yes (III) | No | Yes |
| 8 | 1250 | 27+5 | Male | Partial course | 2 | No | No | Yes |
| 9 | 1480 | 29+1 | Male | Full course | 1 | No | No | Yes |
| 10 | 820 | 25 | Male | Full course | 6 | Yes (III) | Yes | Yes |
| 11 | 980 | 26+6 | Male | Partial course | 3 | Yes (III) | No | No |
| 12 | 840 | 25+5 | Male | Full course | 2 | No | No | Yes |
| 13 | 690 | 27+5 | Female | Full course | 9 | No | No | Yes |
| 14 | 660 | 27+3 | Female | Full course | 9 | No | No | Yes |
| 15 | 1170 | 26+6 | Male | Partial course | 7 | Yes (II) | No | Yes |
Sonographic features of patent ductus arteriosus and treatment characteristics.
| Patient | PDA size (mm/kg) | LA:Ao ratio | Previous ibuprofen treatment | Initiation paracetamol (days of life+weight) | Paracetamol (days) | Indication | Success | Surgery |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.8 | – | Yes (1 dose) | 7, 900g | 3 | IVH+thrombocytopaenia | Yes | No |
| 2 | 5 | – | No | 8, 550g | 6 | IVH | Yes | No |
| 3 | 2.59 | 1.6 | No | 7, 1140g | 7 | IVH | Yes | No |
| 4 | 2.5 | 1.7 | Yes (1 cycle) | 27, 960g | 7 | IVH+treatment failure | No | Yes |
| 5 | 1.6 | 1.6 | Yes (1 cycle) | 14, 1100g | 7 | Treatment failure | Yes | No |
| 6 | 2.75 | 2.7 | No | 3, 880g | 6 | IVH | Yes | No |
| 7 | 3.57 | 2.5 | No | 10, 710g | 7 | IVH | No | Yes |
| 8 | 1.44 | 1.8 | No | 15, 1280g | 7 | Risk of NEC | Yes | No |
| 9 | 1.55 | 2.3 | Yes (2 cycles) | 21, 1760g | 5 | Treatment failure | Yes | No |
| 10 | 3.9 | 1.56 | No | 4, 820 | 6 | Pulmonary haemorrhage+thrombocytopaenia | No | Yes |
| 11 | 2.93 | 1.3 | Yes (2 cycles) | 13, 1680g | 3 | Thrombocytopaenia+sepsis | Yes | No |
| 12 | 2.3 | 2 | Yes (1 cycle) | 19, 1000g | 6 | Sepsis | No | Yes |
| 13 | 2.33 | – | Yes (2 cycles) | 15, 770g | 7 | Sepsis | No | Yes |
| 14 | 2.6 | 1.46 | No | 9, 760g | 3 | Thrombocytopaenia | Yes | No |
| 15 | 2.56 | 1.8 | No | 3, 1770g | 6 | IVH | Yes | No |
Since Hammerman et al.2 discovered by chance that paracetamol can achieve closure of PDA in PTNBs, several studies have been published that propose its use as an alternative treatment in cases where first-line drugs have failed or are contraindicated. Recently, several clinical trials3,4 have shown that paracetamol may be as efficacious as ibuprofen, reporting proportions of PDA closure nearing 75%, somewhat higher than the proportion achieved in our small sample (10/15 patients; 66.7%); however, this approach is used infrequently, almost incidentally, in Spain. Paracetamol is currently proposed as an efficacious and possibly safer alternative to traditional COX inhibitors, whose use is limited due to their contraindications and potential adverse effects (kidney failure, NEC, severe hyperbilirubinaemia, sepsis, coagulopathy, haemorrhage, etc.). The short-term side effects of paracetamol observed to date have been minimal, and paracetamol may have the additional advantage of being more effective in hypoxic states (which are frequent in PTNBs), as it is believed to act on the peroxidase function of COX.5 However, further evidence from clinical trials is required to establish its non-inferiority in comparison to treatment indomethacin or ibuprofen as well as its safety, especially in the long term. On the other hand, PTNBs are at higher risk of liver toxicity due to their immaturity, and the dose of paracetamol used for PDA closure is higher than the dose used for analgesia or reduction of fever (45–60mg/kg/day compared to 30–40mg/kg/day). Furthermore, some case series have found a long-term association of paracetamol use with autism spectrum disorders and neurodevelopmental disorders, and it is recommended that patients treated with this approach are followed up closely with thorough neuropsychiatric evaluations.6 In our small series, all survivors have had adequate psychomotor development, including the two patients that have already reached 2 years of corrected age.
Please cite this article as: Gálvez Criado R, Rodríguez Blanco S, Oulego Erroz I, Jiménez González A, Alonso Quintela P. Paracetamol: tratamiento útil de elección para el ductus arterioso persistente en prematuros de muy bajo peso. An Pediatr (Barc). 2018;88:353–355.