Invasive procedures performed in children, especially in the first year of life, carry a risk of significant vascular injury.1 Numerous cases of pseudoaneurysm secondary to vascular catheterization have been described in the paediatric population.1–4 The mechanism of injury seems to be associated with the small size of the vessels and the movements performed in catheterization attempts, which may tear vessel walls. Failure to apply local compression after catheter insertion is also considered one of the frequent mechanisms of injury.1 Multiple therapeutic options have been suggested to treat post-traumatic pseudoaneurysms in paediatric patients, including ultrasound-guided compression, intralesional thrombin injection,5 endovascular treatment3 and surgical repair.1,2,4
The clinical history of all patients diagnosed and treated for posttraumatic pseudoaneurysm in our center between 2008 and 2021 was reviewed. (Table 1). We contacted the parents of the patients to collect information on their current quality of life, the need of follow-up and the presence of complications.
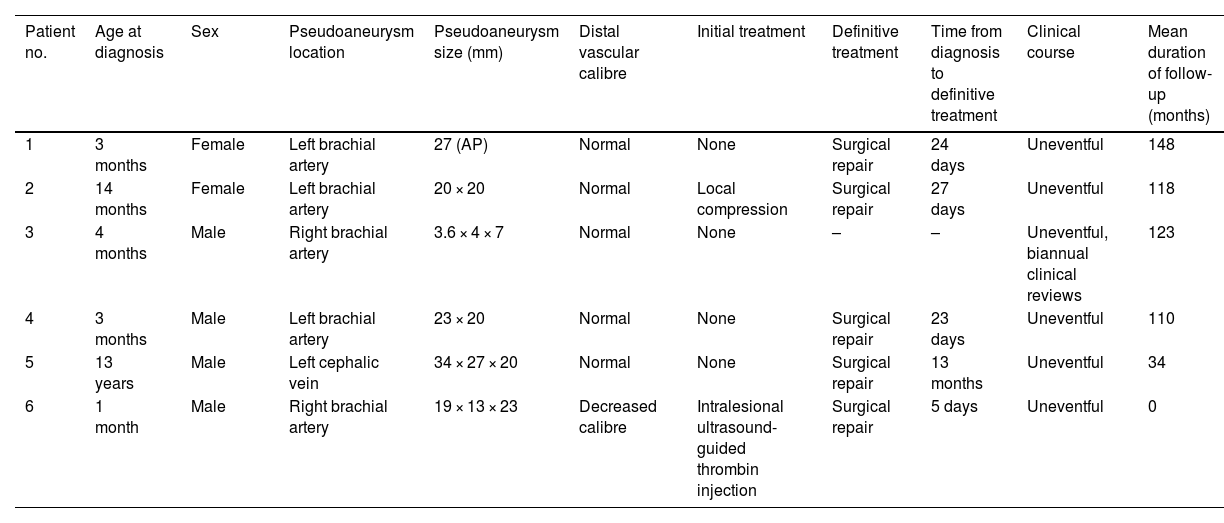
Clinical characteristics of the patients included in the case series.
| Patient no. | Age at diagnosis | Sex | Pseudoaneurysm location | Pseudoaneurysm size (mm) | Distal vascular calibre | Initial treatment | Definitive treatment | Time from diagnosis to definitive treatment | Clinical course | Mean duration of follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 months | Female | Left brachial artery | 27 (AP) | Normal | None | Surgical repair | 24 days | Uneventful | 148 |
| 2 | 14 months | Female | Left brachial artery | 20 × 20 | Normal | Local compression | Surgical repair | 27 days | Uneventful | 118 |
| 3 | 4 months | Male | Right brachial artery | 3.6 × 4 × 7 | Normal | None | – | – | Uneventful, biannual clinical reviews | 123 |
| 4 | 3 months | Male | Left brachial artery | 23 × 20 | Normal | None | Surgical repair | 23 days | Uneventful | 110 |
| 5 | 13 years | Male | Left cephalic vein | 34 × 27 × 20 | Normal | None | Surgical repair | 13 months | Uneventful | 34 |
| 6 | 1 month | Male | Right brachial artery | 19 × 13 × 23 | Decreased calibre | Intralesional ultrasound-guided thrombin injection | Surgical repair | 5 days | Uneventful | 0 |
Between 2008 and 2021, 6 patients received a diagnosis of post-traumatic pseudoaneurysm. Two were preterm infants who needed multiple vascular access lines during their prolonged stay in the neonatal unit. Five were aged less than 1 year at the time of diagnosis of the pseudoaneurysm, and 1 patient was aged 12 years. All patients had a history of traumatic vascular access prior to diagnosis of the pseudoaneurysm. Five had arterial pseudoaneurysms (3 in the left brachial artery and 2 in the right brachial artery), while 1 presented with pseudoaneurysm in the left cephalic vein.
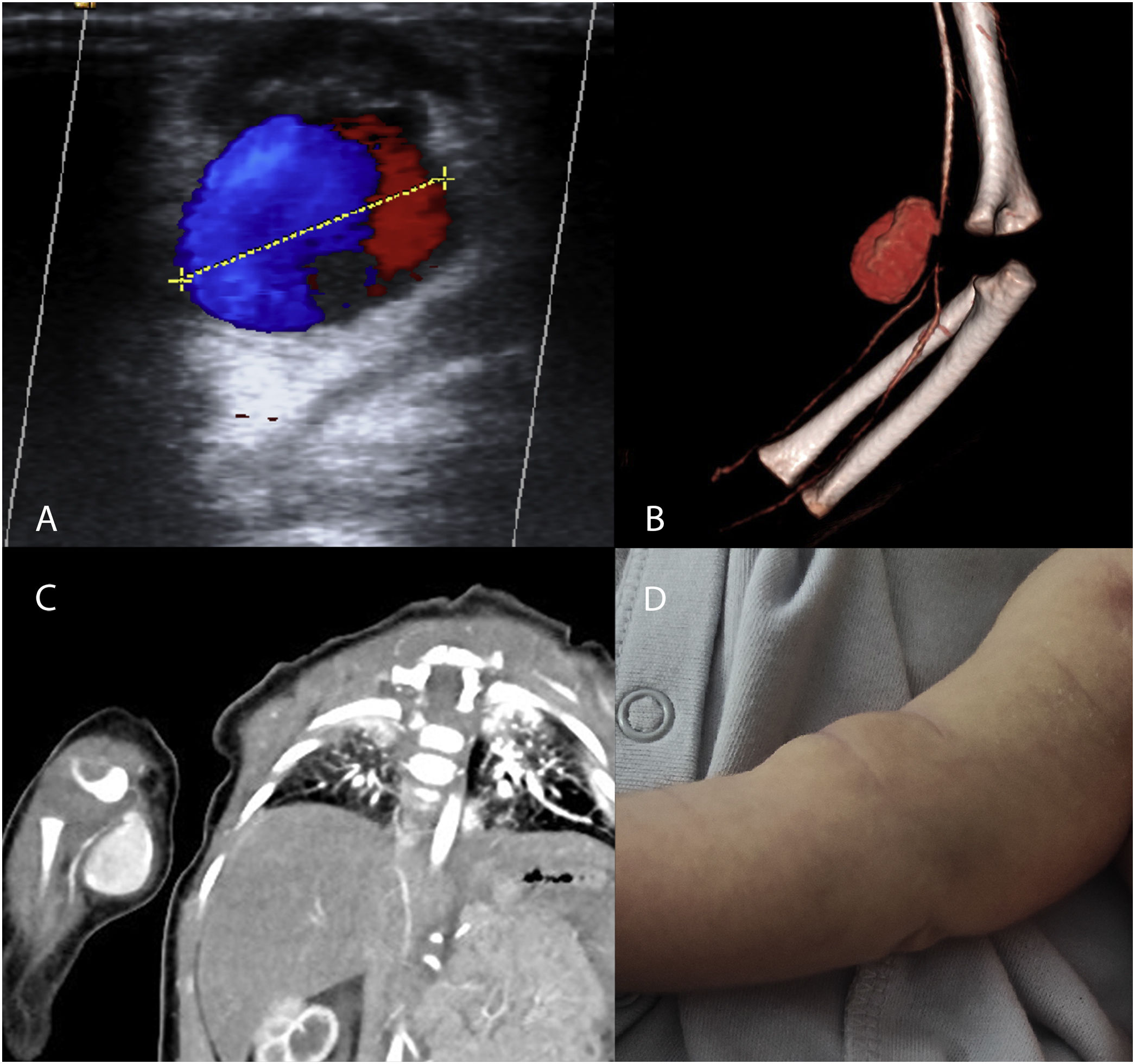
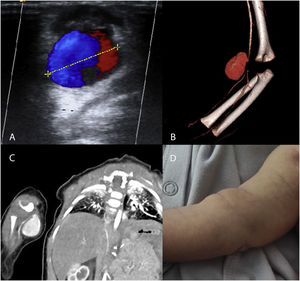
The diagnosis was made by physical examination and Doppler ultrasound (3 patients), computed tomography angiography (CTA, 2 patients) and magnetic resonance angiography (MRA 1 patient) (Fig. 1). One of the patients had filiform stenosis of the brachial artery distal to the pseudoaneurysm. In the remaining 5 patients, the calibre of the vessel distal to the pseudoaneurysm was normal.
(A–C) Imaging studies of patients with pseudoaneurysms. (A) Doppler ultrasound. Yin–yang sign attributed to turbulent flow inside the arterial pseudoaneurysm. (B, C) CTA and 3D reconstruction of pseudoaneurysm dependent on the right brachial artery, with distal narrowing of vessel calibre. (D) Photograph of the patient with a pseudoaneurysm in the right brachial artery. Mass in right forearm 48 h after the traumatic puncture.
One of the patients received mechanical compression and 1 an ultrasound-guided intralesional injection of thrombin before being referred to our hospital. One patient had mild anaemia (haemoglobin [Hb], 9.8 g/dL) at the time of diagnosis.
Five patients underwent surgical repair of the pseudoaneurysm. The selected surgical approach was the opening of the pseudoaneurysm under temporary interruption of local circulation followed by endoaneurysmorrhaphy with a discontinuous monofilament suture. None of the patients required a bypass, nor was there significant vasospasm requiring pharmacological therapy. The mean length of stay was 1.33 ± 1.03 days. None of the patients developed postoperative complications.
The remaining patient was managed with watchful waiting due to the long time elapsed from the formation of the pseudoaneurysm (12 months) and its stable size from the time of diagnosis.
In 2021 we contacted the parents of the patients and confirmed that the patients were asymptomatic and did not have any sequelae. In all patients, the cosmetic outcomes of the surgical scar were good. The mean duration of follow-up was 88.8 ± 53 months. Only the patient managed conservatively continued to require follow-up every 2 years, but the size of his pseudoaneurysm has remained stable to date.
Paediatric post-traumatic pseudoaneurysms are rare but potentially serious lesions. Although they are more frequent in children under 1 year, especially in neonates, post-traumatic pseudoaneurysms can also occur in older patients, as was the case of 1 of the patients in this case series.
Pseudoaneurysm should be suspected in the presence of a progressively growing pulsatile mass following manipulation of a vascular access line, and external compression should be initiated as soon as possible. The diagnosis is confirmed by imaging, and Doppler ultrasound is considered the gold standard. The yin–yang sign is commonly observed in the Doppler ultrasound scan, but its specificity is only moderate.6 Doppler flow imaging showing antegrade flow during systole and retrograde flow during diastole allows diagnosis of arterial pseudoaneurysm (but not venous pseudoaneurysm).
The use of other diagnostic techniques should be adapted to each individual clinical scenario (for instance, if a paediatrics or anaesthesia team capable of performing sedation is available to perform a MRA, or axial multiphase CTA delayed phase imaging is chosen, taking into account the significant exposure to radiation that it entails).
In recent years, new therapeutic options have emerged, such as ultrasound-guided intralesional thrombin injection or endovascular treatment. However, the clinical experience with these modalities in paediatric patients is scarce and should be interpreted with caution given the absence of specific studies supporting their use.
We ought to note that intralesional thrombin injection requires a prior echocardiogram to exclude the presence of an atrial septal defect or patent foramen ovale, in case of possible migration of the injected material (a rare complication, but one that may occur in the case of atrial septal defect). This is especially relevant when the pseudoaneurysm involves the venous territory.
Withholding treatment with close monitoring is a reasonable approach to management in patients with long-standing post-traumatic pseudoaneurysms that have been stable over time, although the experience with this type of case is limited in our hospital.
Although there are no clinical guidelines for the management of post-traumatic pseudoaneurysms in the paediatric population, it is assumed that the risk factors for rupture are similar to those in adult cases of pseudoaneurysm (maximum diameter, morphology, etc.). In our experience, the initial management should be surgical, unless, as happened in one of our patients, there is evidence of long-standing aneurysmal stability, in which case conservative management may be a reasonable option.
Nevertheless, in our experience, surgery performed by an experienced specialist is a curative treatment with optimal short- and long-term results.
One of the main barriers is the lack of surgical training on a highly infrequent and technically complex pathology. Some feasible options to facilitate such training would be experimental microsurgery courses, specific rotations in vascular surgery departments or the use of artificial vascular simulators (with 3D printing, silicone prostheses, etc.).
When it comes to the prevention of post-traumatic pseudoaneurysms, clinicians who treat children, especially infants under 1 year of age, should receive specific training on vascular access management to reduce the risk of iatrogenic lesions.
Please cite this article as: Arredondo Montero J, Román Moleón M, Martín-Calvo N, Antona G, Bronte Anaut M, López-Gutiérrez JC. Pseudoaneurismas postraumáticos pediátricos: nuestra experiencia. An Pediatr (Barc). 2022;97:208–211.