Perinatal asphyxia is an event with far-reaching consequences that can lead not only to the development of neonatal encephalopathy, but also to multiple organ failure (MOF). This ailment may result from the redistribution of blood flow, which would preserve the perfusion of vital organs such as the heart, brain and adrenal glands at the expense of other organs.
The objective of the study was to determine the incidence and aetiopathogenesis of failure in the organs most frequently involved in neonatal MOF following perinatal asphyxia.
We conducted a systematic literature search in the PubMed, Scopus and Cochrane Library databases using the MeSH terms (ischemia AND hypoxia AND multiorgan dysfunction AND neonat*), (asphyxia AND multiorgan dysfunction AND neonat*) and (liver/kidney/digestive OR gastrointestinal/heart injury AND ischemia AND hypoxia AND neonat*). We selected clinical and preclinical studies published after 2000 and excluded case series, letters to the editor, cohort studies without comparison groups and abstracts.
In this study, we found that MOF associated with perinatal asphyxia is a frequent phenomenon with a relevant impact on neonatal morbidity and mortality, as it can cause changes not only in the kidney, liver and gastrointestinal tract, but also cardiomyopathy if the ailment is protracted or severe.
La asfixia perinatal es un acontecimiento con efectos de gran alcance, pudiendo conducir no solo al desarrollo de encefalopatía neonatal, sino también a un fallo multiorgánico (FMO). Esta afectación posiblemente se deba a la redistribución del flujo sanguíneo, mediante el cual se conserva la irrigación de órganos vitales como el corazón, el cerebro y las glándulas suprarrenales, a expensas de su disminución en otros órganos.
El objetivo del presente trabajo fue conocer la incidencia y la etiopatogenia de los órganos más frecuentemente afectados en el FMO neonatal tras la asfixia perinatal.
Se realizó una búsqueda bibliográfica sistemática en las bases de datos Pubmed, Scopus y The Cochrane Library empleando los términos MeSH (ischemia AND hypoxia AND multiorgan dysfunction AND neonat*), (asphyxia AND multiorgan dysfunction AND neonat*) y (liver/kidney/digestive OR gastrointestinal/heart injury AND ischemia AND hypoxia AND neonat*). Se incluyeron trabajos clínicos y preclínicos posteriores al año 2000 y se excluyeron series de casos, cartas al director, cohortes sin grupo comparador y abstracts.
En el presente trabajo describimos que el fallo multiorgánico asociado a la asfixia perinatal es un fenómeno frecuente y relevante en la morbimortalidad del neonato, pudiendo llegar a producir no solo alteraciones en riñón, hígado y tracto gastrointestinal, sino también miocardiopatía si el fenómeno se prolonga o es de elevada gravedad.
The incidence of perinatal asphyxia continues to be relatively high in live births (1‰ to 6‰).1 Perinatal asphyxia refers to a process characterised by progressive hypoxia, hypercapnia and acidosis,2 resulting in a transient but potentially harmful oxygen deprivation. The chief and most severe potential complication is the development of hypoxic-ischaemic encephalopathy (HIE), which, with an incidence of 2.5 per 1000 live births,3 is a severe disease associated with significant morbidity and mortality.4
During asphyxia, to preserve adequate perfusion and oxygenation of the brain, heart and adrenal glands, the cardiac output is redistributed at the cost of reducing the irrigation of other organs, possibly leading to secondary damage in the latter.2,5,6 This is one of the main causes considered in the case of multiple organ dysfunction (MOD) resulting from the interruption of placental flow.2,5 If the flow reduction persists in time, local vasoconstriction may cause cell damage and inadequate tissue function, causing postnatal dysfunction.5,6 Shah et al6 found that all infants that had HIE secondary to severe asphyxia exhibited manifestations of dysfunction of at least one organ or system, in addition to the central nervous system. Thus, perinatal asphyxia and any subsequent MOD may be associated with a high risk of lifelong severe complications that need to be taken into account.7
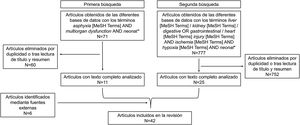
Material and methodsWe conducted a systematic review using the PubMed search engine, which provides access to different literature databases compiled by the National Center for Biotechnology Information in the National Library of Medicine, Scopus and the Cochrane Library. We included clinical and preclinical studies published after 2000 and excluded case series, letters to the editor, cohort studies without a comparison group and summaries or abstracts.
In an initial search, we used asphyxia [MeSH Terms] AND multiorgan dysfunction AND neonat*. We read the abstracts of the search results to determine whether the articles focused specifically on multiorgan dysfunction after perinatal asphyxia, that is, whether they could include relevant data or, on the contrary, they were not suitable to include in the review. We selected 11 articles.
While the selected articles provided a general perspective from which to start developing the review, they did not provide sufficient data on each particular organ, so we decided to do additional searches for each of the organs of interest. The organs selected for this purpose were the liver, kidney, gastrointestinal tract and heart. This selection was based on the frequency with which each of them were affected after perinatal asphyxia and their potential contribution to neonatal morbidity and mortality. We did not include data on central nervous system abnormalities, as brain injury, while very important, is well-known in this context and the purpose of our work was to describe how other organs were affected post asphyxia.
The terms included in the second search on the different organs were liver [MeSH Terms]/kidney [MeSH Terms]/digestive OR gastrointestinal/heart [MeSH Terms] injury [MeSH Terms] AND ischemia [MeSH Terms] AND hypoxia [MeSH Terms] AND neonat*, once again limiting the search to articles published between 2000 and 2021. The process of article selection in this search was the same applied in the previous one, starting with the perusal of the abstracts and, in the case the article focused specifically on the subject of multiorgan dysfunction after perinatal asphyxia and/or included data relevant to the review (incidence, aetiology, pathological/histological correlates and/or biomarkers), reading of the full text. After excluding duplicates already selected in the first search and articles unrelated to the subject under study, and having added articles from external sources, the final selection for the review included a total of 42 articles (Fig. 1). The entire search and result review process was performed in duplicate. Disagreements between authors were resolved by consensus.
Multiple organ dysfunction following perinatal asphyxiaWe now proceed to present the information found in the literature about the organs most frequently affected following perinatal asphyxia.
KidneyIncidenceThe incidence of renal injury in term newborns that experience perinatal asphyxia is substantial (50%–72%), and this complication is associated with an increased risk of mortality. It should be noted that there are no fixed criteria to determine its presence. The usual approach relies on the elevation of urea and creatinine levels associated with a decreased urine output,8 although it is important to remember that creatinine levels do not usually increase until 25%–50% of renal function is lost, and that renal failure is not always accompanied by oliguria.5,8
Kidney injury and its aetiologyDespite the significant incidence of renal injury, few studies have assessed the changes undergone by the kidney. Before birth, the high renal vascular resistance, high activity of plasma renin and low glomerular filtration rate, among other factors, make this organ particularly sensitive to hypoperfusion. The main type of renal injury is acute tubular necrosis and, in more extreme cases, diffuse renal cortical necrosis.8 Prolonged hypoperfusion may not only reduce glomerular filtration, but also damage cells with a higher energy consumption (tubular cells), causing necrosis of the proximal tubule and the ascending loop of Henle. In addition, the infiltration of neutrophils circulating in the ischaemic renal tissue and the release of cytotoxic substances may promote damage not only in endothelial cells but throughout the kidney.9
In an in vitro study of human proximal renal tubule cells subjected to hypoxia followed by reoxygenation, Du et al10 found a lower number of viable cells and an increased lactate dehydrogenase activity. The authors concluded that the integrity of the membrane is compromised by hypoxic episodes and that tubular cells may suffer irreversible damage. In a newborn pig model, Satas et al11 detected the presence of renal infarction secondary to hypoxia-ischaemia.
Based on the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines,12 the AWAKEN study defined acute kidney injury in newborns as an increase in serum creatinine by 0.3mg/dL or greater (26.5μmol/L) or to 1.5 times the baseline value or more, and/or a urine volume of less than 1mL/kg/hour in days 2–7 post birth.13 In this study, the serum creatinine threshold used to define stage 3 kidney injury was 2.5mg/dL (221μmol/L) rather than the 4.0mg/dL (353.6μmol/L) applied in adults.12,13 Predictive tools have also been developed, such as the renal angina index, which aims to identify children at risk of acute kidney injury lasting more than 72h beyond functional impairment. The clinical signs of injury are based on changes in the estimated creatinine clearance or the percentage of fluid overload.14 The neonatal STARZ score, developed recently, is used for quick quantitative assessment of the risk of acute kidney injury in newborns admitted to the neonatal intensive care unit (NICU) by means of variables such as age at admission, gestational age, sepsis, significant cardiac disease, urine output, serum creatinine and the use of nephrotoxic drugs, furosemide or inotropes.15
When it comes to newborns with neonatal encephalopathy treated with therapeutic hypothermia, there is substantial heterogeneity in renal function and injury. Some authors have asserted that serum creatinine values relative to postnatal age may provide relevant information for the management of these infants.16 Other studies have assessed the predictive value of several urine markers of acute kidney injury: while the levels of neutrophil gelatinase-associated lipocalin, kidney injury molecule-1 and interleukin-18 were found to be elevated in newborns with HIE managed with therapeutic hypothermia who developed acute kidney injury, due to the substantial variability in their levels and poorly defined thresholds their clinical usefulness is unclear.17
Electrolyte imbalancesAsphyxiated newborns are at risk of developing hyponatraemia secondary to oliguric acute kidney injury due to water retention and a decrease in sodium reabsorption at the tubular level.8 They may also develop syndrome of inappropriate antidiuretic hormone, which can contribute to the development of hyponatraemia, considerable increase in weight, decreased serum osmolality considerable, oliguria and/or increased urine osmolality.2,8
An important aspect to consider in these newborns at higher risk of hyperkalaemia due to renal failure is the increase in cardiac excitability.8 This cardiac abnormality can be exacerbated by hypocalcaemia.2 Magnesium levels may also be abnormal, either reduced or elevated,8 with evidence of low levels in the polyuric phase of acute tubular necrosis.2
LiverLiver damage seems to be mostly associated with hypoperfusion secondary to blood flow redistribution as opposed to hypoxia itself,5 as the decreased perfusion of the liver parenchyma, especially in the right lobe,18 can cause hypoxic hepatitis.19
IncidenceThere is evidence of an association between perinatal asphyxia and liver damage, although the reported incidence varies widely (22%–80%) (Table 1). Hankins et al20 reported the highest incidence (80%), although this figure could be related to the severity of the cases in the study, as patients had to have developed encephalopathy after the asphyxia episode to be included. In a later study,21 nearly 50% of newborns had not developed HIE. However, both studies applied less stringent criteria to define liver damage compared to other studies, like the one by Tarcan et al.,22 in which the levels of alanine aminotransferase had to exceed 100 U/L, or the one by Barnett et al.,23 in which the peak values of alanine aminotransferase or aspartate aminotransferase had to be 200 U/L or greater at 48h. Possibly as a result of this, the incidence reported in these studies was of 39% and 22%, respectively. Furthermore, the latter one included neonates that had died of perinatal asphyxia, which is indicative of the severity of the asphyxia event in the neonates included in the sample.
Variation in the incidence of liver damage between studies.
| Reference. | Incidence | Sample | Biomarkers | Criteria for liver damage |
|---|---|---|---|---|
| Type of study | ||||
| Choudhary et al., 2015 | 42.85% | 14.28% HIE stage I, | ALT, AST, AP, LDH, total protein, serum albumin, PT, INR | ALP>50U/L, |
| Prospective case (n=70) and control (n=30) study in newborns | 25.73% HIE stage II, | AST>140U/L, | ||
| 11.42% HIE stage III, | AP>420U/L, | |||
| 48.57% normal | LDH>580U/L, | |||
| Total protein<4.5g/dL, | ||||
| Serum albumin<2.5g/dL, | ||||
| PT>20s | ||||
| And/or INR>1.2 | ||||
| Karlsson et al., 2009 | 63.16% | 45-min global hypoxia-ischaemia insult followed by 72-h survival | 4 histological sections from standardised areas in the liver | Damage observed in the histological section |
| Preclinical model in pigs (n=19) | ||||
| Tarcan et al., 2007 | 39% | Newborns admitted to the neonatal intensive care unit with a diagnosis of perinatal asphyxia | ALT | Elevation of ALT to twice the upper limit of normal (approx.>100U/L) |
| Retrospective cohort study in newborns (n=56) | ||||
| Karlsson et al., 2006 | 46% | Asphyxiated term newborns with 5-min Apgar score <7 | AST, ALT, LDH, GGT, albumin, total and conjugated bilirubin, cholinesterase activity, INR, blood cell counts | Elevation of AST or ALT more than 2 standard deviations above the mean of the control group, peaking more than 24h after birth, and subsequent decrease to values near normal at 10 days |
| Case (n=26) and control (n=56) study in newborns | ||||
| Barnett et al., 1997 | 22% | Deceased after perinatal asphyxia | ALT, AST | Peak ALT or AST values≥200U/L in the first 48h post birth |
| Retrospective cohort study in newborns (n=58). | ||||
| Hankins et al., 2002 | 80% | Newborns had to have encephalopathy post asphyxia | AST, ALT, LDH | AST or ALT or LDH value≥1.5 times the value in control group |
| Prospective cohort study (n=46) |
The table presents the reference for each article, the type of study, incidence, main sample inclusion criteria and the markers and criteria for definition of liver used in each study. ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; INR, international normalised ratio; LDH, lactate dehydrogenase; PT, prothrombin time.
This variation in the incidence could be explained not only by discrepancies in the definition of liver damage, but by the inclusion criteria applied in each study. It is also worth noting that liver damage may be underdiagnosed if liver markers are used for its identification, for, while there is a correlation between liver enzyme levels and liver damage,21 normal levels of transaminases or lactate dehydrogenase do not rule out damage.
Histological correlates of liver damageIn a study of 19 newborn pigs, Karlsson et al.24 found foci of necrosis in 5 animals, infarction in 6, neutrophil infiltration in 6, thrombi in the intrahepatic portal veins in 3 and subcapsular haematoma in 1. Ikeda et al.18 reported detection of cytoplasmic eosinophilia and canalicular cholestasis in samples obtained from asphyxiated lambs and fatty changes in tissue samples from lambs that suffered mild asphyxia. Two brain-damaged lamb foetuses had fatty changes and congestion in the liver, in addition to inflammatory cell infiltration and centrilobular necrosis.
In the pathological examination of infants that died of post-asphyxial HIE, Barnett et al.23 detected congestion in 22, fatty changes in 13, haemorrhage in 4, centrilobular necrosis in 7 and other necrotic-ischaemic changes in 2.
Although there is evidence that progression to complete hepatic failure is rare,21 there is also evidence that hepatic involvement is associated with a high mortality.22
Coagulation abnormalitiesIn infants that have experienced perinatal asphyxia, the prothrombin time and the international normalized ratio are significantly elevated,19,21 and there is a risk of haemorrhage if there are additional alternations, such as a lower platelet count or decreased fibrinogen level.5
The liver synthesises a large part of the proteins involved in coagulation, but the mechanism by which hypoxia leads to bleeding is not well understood. Thus, it may be reasonable to expect coagulation disturbances if liver function is impaired.11 However, Chadd et al.,25 in a case-control study that included 47 term newborns, found that neonates that had experienced hypoxia exhibited coagulation abnormalities compared to controls, and that the source of the disturbance was the consumption of clotting factors, that is, disseminated intravascular coagulation. Similar results have been described by Chessells and Wigglesworth26 in 9 infants that suffered severe hypoxia, suggesting that direct liver damage would play a secondary role.
Gastrointestinal tractIncidencePerinatal asphyxia may result in decreased perfusion of the gastrointestinal tract, manifesting with vomiting, diarrhoea, gastrointestinal haemorrhage and even necrotising enterocolitis (NEC).4 The incidence of these events is 29%.27
The intestine is one of the organs most sensitive to ischaemia,27 as it has areas located between two major arteries (mesenteric arteries) that are prone to injury secondary to perinatal asphyxia.5 In addition, intestinal injury is not caused only by ischaemia, but also by reperfusion.27
In addition to an underdeveloped regulation of vascular resistance, the immature intestine has increased metabolic demands. This can cause problems in episodes of cardiovascular stress, as the immature body may not be able to increase intestinal blood flow and metabolic demands may overwhelm the infant’s ability to increase oxygen consumption. This results in defective pressure flow autoregulation in response to hypotension, leading to tissue hypoxia.28 Thus, gastrointestinal complications are among the chief disturbances present in the context of perinatal asphyxia, with a pattern of intestinal injury similar to that found in NEC.29
Necrotising enterocolitisNecrotising enterocolitis is a potentially devastating acquired intestinal disorder in preterm infants. Its incidence in very low birth weight infants is 7%, and it is associated with a high mortality (15%–30%) and substantial morbididy.29–32 It is characterised by intestinal inflammation and ischaemia and changes in intestinal microcirculation.35,36
Aetiology and pathogenesisThe aetiology of NEC is not well established, although it seems that the main risk factors are enteral feeding, pathogen colonization and systemic hypoxia, among others, triggering a severe inflammatory response that affects the immature intestinal epithelium of the neonate.28,33 Enteral feeding may increase oxygen demands past the available supply in the neonatal intestine, resulting in partial hypoxia. There are reports of cases of NEC associated with ischaemic events, which, combined with the increased frequency of this disorder in areas of the intestine that are more sensitive to ischaemia due to their location between the inferior and superior mesenteric arteries (such as the distal ileum and proximal colon), suggests that this disorder is associated with circulation abnormalities.28,33
In a rat model, Balyemez et al30 concluded that the observed microscopic lesions were virtually the same as those described in neonatal NEC: severe destruction of villi and crypts in the intestinal mucosa.
HeartWhen the interruption of placental blood flow is severe or prolonged and compensatory mechanisms are overwhelmed, the decreased cardiac output and the drop in the mean arterial pressure result in decreased blood flow in the brain and organ systems, increasing the likelihood of ischaemic injury.2,34,35
Aslam and Molloy36 included the heart among the organs most frequently affected by asphyxia, likely due to the immaturity of muscle tissue in the newborn, which would result in an inadequate response to the asphyxia event. In addition, cardiac dysfunction further complicates the already compromised circulatory status.35
IncidenceHypoxic-ischaemic myocardial injury is increasingly contemplated as a potential cause of cardiovascular dysfunction in newborns, as it is present in up to one third of affected infants and could contribute to an increase in neonatal morbidity and mortality.7,37 Hankins et al.20 conducted a cohort study on acute perinatal asphyxia followed by encephalopathy and found that 78% of cases were associated with cardiac injury, defined as inotrope requirement after 2h post birth and/or elevation of creatinine kinase-MB. Meanwhile, Shah et al.6 found an incidence of 62%, in contrast to the 29% established by Martín-Ancel et al.27
Markers of cardiac damageWhen the heart is affected by the asphyxia event, a transitory myocardial ischaemia may develop that is known as hypoxic cardiomyopathy. A cohort study was conducted to determine the frequency of this disorder in newborns with cardiovascular symptoms, finding an incidence of 70% in newborns with perinatal asphyxia.38
One of the markers used to assess for the presence of myocardial injury is creatine kinase-MB.14,37,39 However, other authors consider that this biomarker offers a low specificity for in newborns, as enzyme levels decrease considerably from 24h after the asphyxia event.38 Therefore, normal values of this isoenzyme do not reliably rule out myocardial injury.27 Other sensitive indicators are troponin-T, troponin-I and brain natriuretic peptide.39,40
Using these markers and other diagnostic methods, such as electrocardiography chest radiography, makes early detection of myocardial injury possible. This is of utmost importance, as evidence has emerged that transitory myocardial ischaemia secondary to perinatal asphyxia is more frequent than previously expected.38 Early diagnosis of myocardial injury can help guide management, thus improving neonatal outcomes.38,40
Cardiac functionIn an observational study in neonates with severe asphyxia, Barberi et al.37 found that, in addition to elevation of cardiac enzymes and relevant ischaemic electrocardiographic changes, patients also had depressed ventricular function.
Hochwald et al.41 compared left ventricular cardiac output in neonates with HIE and healthy neonates, and found a decrease of 42.34% in the former. Gurgul et al.34 found that in newborn rat pups, contractile force was only affected in the ventricles.
Mitochondrial abnormalitiesThe incidence of myocardial injury established based on histological findings after perinatal asphyxia appears to be higher compared to estimates based on clinical manifestations. Gurgul et al.34 examined cardiac cells with electron microscopy and found degenerative changes in some myocardial cells that exhibited thinning and rupture of myofibrils, as well as degenerative mitochondrial changes. Yang et al.42 demonstrated that these alterations in mitochondria were reflected in their function, as the mitochondrial respiratory chain in myocardial cells was affected, probably due to an intracellular calcium overload.
ConclusionAsphyxia is a phenomenon with a significant prevalence. One of the most frequent complications secondary to asphyxia is MOD, and volume redistribution is its main cause. Prolonged and/or severe episodes of perinatal asphyxia may cause cardiomyopathy.
There are difficulties that pose barriers to establishing a narrow incidence range for the involvement of each organ in the context of MOD in perinatal asphyxia. This is why the establishment of standardised criteria is considered necessary to homogenise the results of different studies.
Future perspectivesEstablishing definitions for dysfunction for each organ and criteria to diagnose MOD would be useful, as there is significant variability in their reported incidence in the studies that focus on the subject. Also, more studies are required to determine the incidence of the observed gastrointestinal complications.
With the evident association between perinatal asphyxia and MOD, having been demonstrated, it may be interesting to delve into the specific management of each affected organ in asphyxiated neonates, and its performance.
It would be interesting to investigate how therapeutic hypothermia affects different organs, as, at present, it is the only authorised treatment effective in reducing the sequelae of HIE after perinatal asphyxia.
Although there are significant differences in anatomy and physiology between male and female individuals, and differences in severity and response to treatment based on sex have already been described in other diseases, there is a dearth of data on the effect of sex on MOD.
FundingThe study was funded by grants from the EITB Maratoia-BIOEF (BIO18/IC/003) and the Spanish Ministry of Science and Innovation (MINECOR20/P66/AEI/10.13039/501100011033).
Conflict of interestsThe authors declare that they have no conflict of interest.