We present the case of a male adolescent aged 1 year with asthma diagnosed at 7 years referred to the department of pulmonology because, since age 12 years, he had trouble breathing within 5 min of engaging in moderate physical activity, accompanied my malaise, nausea and sweating. The dyspnoea was not accompanied by cough or any other breathing sounds and improved only gradually after ceasing physical activity, with no improvement with inhaled salbutamol. The patient also reported precordial catch during physical activity, starting 6 months prior, which was followed by sudden syncope on 2 occasions, with spontaneous recovery within 5 min and with normal vital signs in the assessment by emergency medical team. The dyspnoeic episodes could not be controlled at the primary care level with escalation of treatment up to step 4 in the Spanish asthma management guidelines (GEMA).
The patient underwent assessment with a free running asthma test, during with the reported symptoms recurred at 5 min in the 140–150 bpm range, with shortness of breath in the absence of cough, wheezing, desaturation or syncope. The patient was referred for a cardiovascular evaluation, in which the physical examination, electrocardiogram and echocardiogram were normal, but the findings of the stress test suggested coronary ischaemia in the region of the anterior descending artery (ADA) (Fig. 1, top) and the CT coronary angiogram evinced the presence of myocardial bridging (MB) at the ADA (Fig. 1, bottom). The coronary angiography without pharmacological spasm provocation was normal. It appeared that MB type B (Schwarz classification)1 could be causing the symptoms, so treatment with bisoprolol was initiated at a dose of 5 mg/day, and the patient was advised to avoid situations resulting in adrenergic stress, which achieved full resolution with normal findings in follow-up stress tests the year after diagnosis. The patient maintained control of asthma with inhaled steroids at medium doses, and was advised to prioritise the use of ipratropium bromide during exacerbations, in addition to administration of salbutamol under monitoring in health care centres.
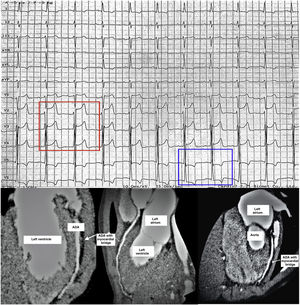
Top. Electrocardiogram conducted at the time the exercise challenge test was interrupted due to chest pain 8 min after starting. ST segment elevation measuring 3 mm in V2–V3 leads and 1 mm in V4 (red rectangle around V2–4), and 1–2 mm ST segment depression with T wave inversion in leads V5 and V6 (blue rectangle around V5–6), compatible with ischaemia in the region supplied by the anterior descending artery (ADA). The pattern normalised completely after 4 min of rest.
Bottom. Three different views from the same coronary CT angiography showing myocardial bridging, 8 mm in length and 4.5 mm in width, in the medial segment of the ADA (white arrows). The course of the ADA leaves the surface of the heart to tunnel into the myocardium of the left ventricle in the different views. The rest of the coronary anatomy was normal.
Myocardial bridging is a congenital anomaly in which the coronary artery (70% of the interventricular branch of the ADA) takes an intramyocardial course and can be compressed during the heart cycle, leading to manifestations of acute coronary syndrome (ACS).2,3 Myocardial bridging may cause exercise-induced dyspnoea and coexist, as was the case of our patient, with a more frequent aetiology like asthma, so it is essential to investigate clinical variables that can guide the differential diagnosis (Table 1). This is crucial in the case of coronary anomalies, in which the use of beta blockers for treatment of asthma exacerbations could trigger or exacerbate ACS.
Clinical variables to consider in the differential diagnosis of exercise-induced bronchospasm secondary to asthma and cardiovascular causes of dyspnoea during exercise. Possible aetiologies of exercise-induced dyspnoea.
| Exercise-induced bronchospasm | Cardiovascular aetiology | |
|---|---|---|
| Family/personal history | Atopy, asthma | Sudden death, arrhythmia, or cardiovascular disease in relative aged <50 years |
| Onset of symptoms | At 5–10 min of starting activity, persisting for 30 min | A few minutes after starting the activity |
| Resolves at rest | ||
| Suggestive symptoms | Shortness of breath | Precordial catch syndrome. |
| Dry cough | Vegetative symptoms: malaise, nausea, sweating, pallor | |
| Chest pain | Palpitations | |
| Syncope or presyncope | ||
| Physical examination | Wheezing, tachypnoea, hypoventilation | Murmurs |
| Arrhythmic heart sounds | ||
| Treatment with bronchodilators | Improvement of symptoms | No response or worsening of symptoms |
The clinical relevance of MB is still under debate, and we cannot be certain whether it was the true cause of the symptoms experienced by our patient. It appears to be a benign lesion, given its frequent detection in asymptomatic patients, especially in association with hypertrophic cardiomyopathy (28%), and it is not associated with an increased risk of sudden death, and it is an exceptional finding in patients without heart disease.4 However, while rare, severe cases associated with ACS in healthy patients have been reported.5 This controversy can be explained by the lack of standardised management, and it is mainly diagnosed based on the visualization of systolic vascular collapse during drug provocation tests with conventional angiography.2,3 The growing availability of multidetector CT has evinced its increased sensitivity in the detection of smaller and more superficial MBs in which the vascular collapse could go undetected, especially in young patients without cardiovascular risk factors.6 Therefore, we believe that in cases like the one presented here, of MB detected with CT angiography but not in the coronary angiography, it is better not to rule it out as a possible cause of ACS, especially in the absence of a clear aetiology. On the other hand, the detection of MB did not rule out the diagnosis of asthma in our patient. It would, however, explain the progressive worsening and the clear association with physical exertion, which were refractory to the escalation of asthma treatment. In addition, the progressive severity of the dyspnoea and chest pain in our patient, even with occasional syncope from age 12 years, could be explained by the gradual and physiological myocardial enlargement and the increased burden placed during physical activity from puberty, resulting in an increasingly severe compression of the coronary artery.1,2
The management of MB is based on the Schwartz classification, which correlates to the development of cardiovascular adverse events during the follow-up.1 Type A (asymptomatic, incidental finding) would not require treatment. Types B (symptomatic with signs of ischaemia on exertion) and C (symptomatic with detection of vascular collapse on angiography) benefit from treatment with beta blockers due to its inotropic and negative chronotropic effect. Surgical revascularization would be reserved for cases refractory to medical treatment. In this case, we opted for a cardioselective beta-blocker to prevent bronchospasm combined with lifestyle changes (avoidance of competitive sports and exceeding a heart rate of 140 bpm in recreational sports) and minimization of the use of beta blockers during asthma exacerbations. Calcium channel antagonists would be an adequate alternative. We ought to highlight the importance of coordinating care between paediatric specialities to ensure adequate management of comorbidities managed with blockers, as was the case of our patient, and the need to elucidate the relationship between MB and ACS to improve the empiric management of this frequent coronary anomaly.