Neonatal hyperbilirubinaemia is a common condition in newborn infants. It is estimated that approximately 60%–80% of term newborns and up to 80%–90% of preterm newborns develop some degree of jaundice. However, although most cases are mild and do not require treatment, the early detection and management of jaundice is essential to prevent complications.1
Given the high incidence of this condition, small modifications to diagnostic and therapeutic protocols can be associated with a decrease in the use of diagnostic tests or treatments.
In September 2022, the American Academy of Pediatrics (AAP) published a revision of its clinical practice guidelines on the management of hyperbilirubinaemia in the newborn infant 35 or more weeks gestation published in 2004.2 This revision, among other things, changed the thresholds for the treatment of hyperbilirubinaemia and established a clear basis for its management: risk groups, indication of venous blood collection for measurement of serum bilirubin based on transcutaneous bilirubin values, interval between follow-up assessments the appropriate timing for initiation and discontinuation of phototherapy or other treatments.
Our hypothesis was that the implementation of a new protocol based on the latest guidelines would result in a reduction in the number of blood draws and admission for phototherapy in the maternity ward of our hospital without an accompanying increase in the short-term neurologic complications or hearing loss associated with hyperbilirubinaemia.
We conducted a retrospective, observational and inferential cohort study.
The revised guidelines of the AAP from 2022 were introduced in our maternity ward in December of the same year. Thus, we compared 2 periods: October-November 2022, before implementation, and February-March 2023, after implementation.
The study universe corresponded to the neonates born at or after 35 weeks of gestation with birth weights greater than 2kg admitted to the maternity ward of a tertiary care hospital.
The data were analysed with the statistical package R version 4.3.1 for Windows. We compared continuous variables with the Student t test and qualitative variables with the χ2 test.
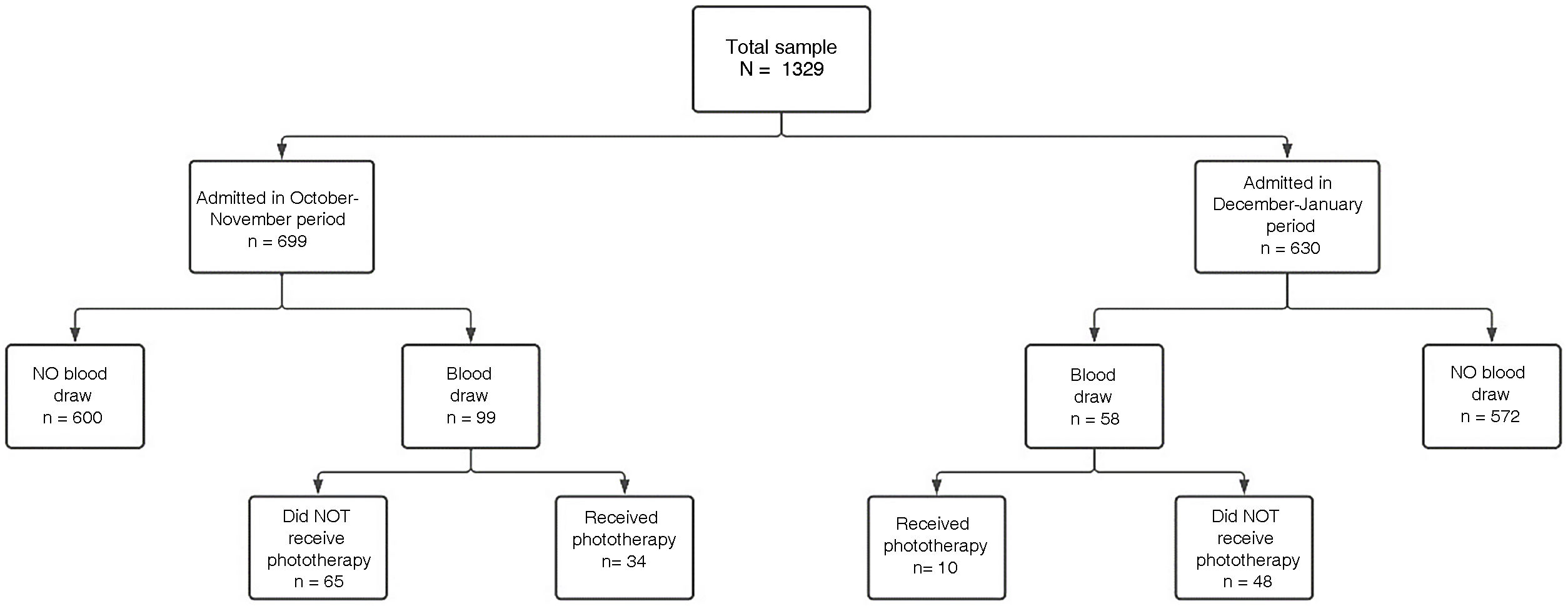
The total number of newborns hospitalised in the maternity ward in the study periods was 1329. Of this total, 699 were admitted in the first period and 630 in the second (Fig. 1).
We assessed neurotoxicity risk factors in both cohorts to determine whether the two groups were comparable. The risk factors considered for this purpose were preterm birth and a positive direct Coombs test, and we did not find statistically significant differences between the periods.
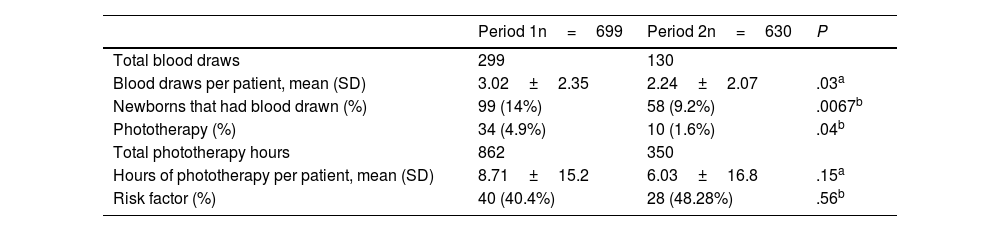
In the first period, a total of 299 blood draws were performed, compared to 130 in the second. The number of neonates that underwent blood draws for measurement of serum bilirubin was 99 in the first period (14% of admitted newborns) compared to 58 in the second (9.2 of admitted newborns). The number of newborns managed with phototherapy was 34 in the first period compared to 10 in the second period, for a total of 862h of phototherapy in the first period compared to 350 in the second period (Table 1).
Baseline characteristics and comparison with the second period after the implementation of the new management protocol.
| Period 1n=699 | Period 2n=630 | P | |
|---|---|---|---|
| Total blood draws | 299 | 130 | |
| Blood draws per patient, mean (SD) | 3.02±2.35 | 2.24±2.07 | .03a |
| Newborns that had blood drawn (%) | 99 (14%) | 58 (9.2%) | .0067b |
| Phototherapy (%) | 34 (4.9%) | 10 (1.6%) | .04b |
| Total phototherapy hours | 862 | 350 | |
| Hours of phototherapy per patient, mean (SD) | 8.71±15.2 | 6.03±16.8 | .15a |
| Risk factor (%) | 40 (40.4%) | 28 (48.28%) | .56b |
SD, standard deviation.
We found statistically significant differences in the total number of blood draws, the number of newborns that had blood drawn and the number of newborns admitted for phototherapy, all of which were lower in the second period. However, we did not find statistically significant differences in the hours of phototherapy between the two periods.
When we analysed short-term complications in each period, we found that none had occurred in either.
The changes to the hyperbilirubinaemia management protocol based on the guidelines of the AAP of September 2022 were associated with an improvement in care quality. They achieved a significant reduction in the number of diagnostic tests and painful procedures performed in newborns, the length of stay, the number of phototherapy treatments and the separation of mother and child, without an associated increase in complications, at least in the short term. The main limitation of the study was the short duration of follow-up. Additional studies need to be performed in the future to assess the long-term impact complications associated with the implemented changes to be able to confirm and broaden our conclusions.